A new era for ceramic matrix composites - cermet insert manufacturer
where \({\rm{\Delta }}{{G}_{dis}}^{{\rm{o}}}\) is the free energy change for carbide dissolution in a current-free system, \({\rm{\Delta }}{{G}_{dis}}^{e}=\frac{{\sigma }_{{matrix}}-{\sigma }_{{carbide}}}{2{\sigma }_{{matrix}}+{\sigma }_{{carbide}}}k{j}^{2}V\) is the energy change due to the passage of pulse current through the specimens22. k is the geometric factor, j is the current density, and V is the volume. The higher conductivity of ferrite matrix (\({\sigma }_{{matrix}} > {\sigma }_{{carbide}}\)) results in \({\rm{\Delta }}{{G}_{dis}}^{e} > 0\), which will cause the carbides to be unstable and facilitate the dissolution of carbides into the ferrite matrix as an driving force. Additionally, for the contribution of \({\rm{\Delta }}{{G}_{dis}}^{{\rm{o}}}\), the interfacial energy contribution plays an important role and can be approached by \({\rm{\Delta }}{{G}_{{dis}}}^{{inter}}=\gamma {V}_{m}\frac{dA}{d{V}_{{carbide}}}\)26. Where y, Vm, A and Vcarbide are the specific interfacial energy, the molar volume of carbide, the surface area between carbide and matrix, and the carbide volume, respectively. It can be inferred that the increased surface to volume ratio of matrix/carbide (especially at the tip region of the deformed carbide) will enhance the interfacial energy contribution to the thermodynamically carbide dissolution. Accordingly, the partial dissolution (Fig. 2) potentially results from the lower thermodynamic dissolution barrier for the deformed carbide with a higher surface to volume ratio of matrix/carbide.
Chen, W., Fan, Z., Pan, X. & Bao, X. Effect of confinement in carbon nanotubes on the activity of Fischer-Tropsch iron catalyst. J. Am. Chem. Soc. 130, 9414–9419 (2008).
Rao, K. R. P. M. et al. Mössbauer study of iron Fischer-Tropsch catalysts during activation and synthesis. Energy Fuels 10, 546–551 (1996).
Ben, D. D. et al. Rapid hardening of AISI 4340 steel induced by electropulsing treatment. Mater. Sci. Eng. A. 725, 28–32 (2018).
Sauvage, X., Copreaux, J., Danoix, F. & Blavette, D. Atomic-scale observation and modelling of cementite dissolution in heavily deformed pearlitic steels. Philos. Mag. A. 80, 781–796 (2000).
Yu, G. et al. FexOy@C spheres as an excellent catalyst for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 132, 935–937 (2009).
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker D8 powder diffractometer equipped with a Cu-Kα source operated at 40 kV and 40 mA and a Vantec-1 detector.
Yu, Y. & Gao, G. Lennard-Jones chain model for self-diffusion of n-Alkanes. Int. J. Thermophys. 21, 57–70 (2000).
The nano-indentation tests are carried out to investigate the hardness of ferrite without and with EPT. As shown in Fig. 5(a), the indentation has been confirmed to be applied on the ferrite matrix and then observed by AFM. The statistical results in Fig. 3(b) show that the average hardness of the ferrite matrix distinctly decreases after EPT regardless of the indenter load, which may be attributed to the recovery of dislocation and occurrence of rapid recrystallization during the EPT (Fig. 4(d))18.
57Fe Mössbauer spectroscopy of ε-Fe2C@graphene-C provided further evidence for the formation of ε-Fe2C after the carburization (Fig. 2b and Supplementary Table 1). Moreover, after 100 h reaction in syngas at 573 K (denoted as ε-Fe2C@graphene-S), the ε-Fe2C content remained constant (about 62.8%), indicating the stabilizing effect by the graphic carbon layer on ε-Fe2C even under reaction conditions (Fig. 2b and Supplementary Table 1).
Previous studies22,23 have shown that the EPT can lead to the dissolution of second-phase below the thermodynamic dissolution temperature within a very short time. Due to the thermal effect of EPT, the real temperature of the treated specimens may have approached or exceeded the critical thermodynamic dissolution temperature. In the meantime, with the enhance of the athermal effect of EPT, the thermodynamic barrier decreases and then the dissolution behavior of the second phase occurs. In this work, considering that the temperature rise induced by joule heating only reached 532 °C, a temperature far away from the critical dissolution temperature of carbides in M50 bearing steel24, the complete dissolution of carbides will not be expected. However, the refinement behavior of carbides in the matrix are surprisingly discovered (Fig. 3), which have been rarely reported so far and will be further discussed.
Wang, P. et al. Synthesis of stable and low-CO2 selective ε-iron carbide Fischer-Tropsch catalysts. Sci. Adv. 4, eaau2947 (2018).
Herranz, T. et al. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 243, 199–211 (2006).
Hosoi, A., Nagahama, T. & Ju, Y. Fatigue crack healing by a controlled high density electric current field. Mater. Sci. Eng. A. 533, 38–42 (2012).
Watté, P., Van. Humbeeck, J., Aernoudt, E. & Lefever, I. Strain ageing in heavily drawn eutectoid steel wires. Scr. Mater. 34, 89–95 (1996).
In order to further verify the partial dissolution behavior of carbides, the XRD patterns for the CR specimens with and without EPT are obtained, as shown in Fig. 6(a). According to the Gaussian fitted curves of (110)α diffraction peak (as insert in Fig. 6(a)), the values of FWHM can be measured (Table 2) and shows a distinct decrease after EPT, which therefore verifies the dislocation recovery as observed in Fig. 4. Moreover, it can be clearly seen that the ferrite diffraction peak shifts to smaller angle after EPT. This indicates that the lattice parameter of α-Fe increases after EPT. The carbon content in the ferrite can be estimated based on the relationship between the lattice parameter and carbon constant of α-Fe given by Fasiska and Wagenblast31 as follow: \({a}_{\alpha }(nm)=(0.28664\pm 0.0001)\,\)\(+\,(0.84\pm 0.08)\times {10}^{-3}\cdot {[C]}_{\alpha }\). where aα is the lattice parameter of α-Fe, and [C]α is the carbon content in α-Fe (at.%). Based on the measured lattice parameters, the carbon content in α-Fe before and after EPT is calculated and listed in Table 2, where it can be seen that the carbon content in α-Fe increases substantially after EPT. Obviously, compared to the carbon content in equilibrium ferrite (the solid solubility of carbon in ferrite at 727 °C is 0.0218 wt.%32), the determined carbon content in ferrite after EPT is supersaturated. This result clearly confirms the migration of carbon from carbides to ferrite and partial dissolution of carbides during EPT.
Xu, J., Yang, Y. & Li, Y. Fischer-Tropsch synthesis process development: steps from fundamentals to industrial processes. Curr. Opin. Chem. Eng. 2, 354–363 (2013).
TEM observation of the microstructure from the CR specimens (a) without and (d) with EPT, (b,c) HRTEM observation of the dislocation tangle around carbide in the CR specimens.
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
In summary, through utilizing the designed EPT processing technology, the extensive voids healing and carbide refinement can be realized within a short time (millisecond level). The generation of thermal compressive stress and acceleration of atoms diffusion will together contribute to voids healing process of cold rolled M50 bearing steel. As well, for the deformed Cr-rich carbide with a higher surface to volume ratio of matrix/carbide, the partial dissolution and refinement of carbides could easily occur due to the decreased thermodynamic dissolution barriers and accelerated kinetic diffusion of carbon atoms towards dislocations during EPT. Moreover, this partial dissolution behavior will result in carbon enrichment in ferrite and accelerate the transformation from ferrite to austenite during subsequent heating.
Webster, C. E., Drago, A. R. & Zerner, M. C. Molecular dimensions for adsorptives. J. Am. Chem. Soc. 120, 5509–5516 (1998).
Kresse, T. et al. Influence of supersaturated carbon on the diffusion of Ni in ferrite determined by atom probe tomography. Scr. Mater. 69, 424–427 (2013).
Fischer–Tropsch synthesis was performed in a stainless steel fixed-bed reactor (i.d. = 12 mm). Catalysts were diluted with inert SiC particles in a mass ratio of 1:10 prior to testing. The diluted catalysts (5.5 g) were pre-treated in flowing H2 (10 mL min−1) at 623 K for 3 h before reactions. The pre-treated catalysts were cooled to 373 K in flowing H2 before the introduction of syngas (H2/CO = 1). The reaction temperature was then ramped slowly to 593 K. The permanent gases (H2, CO, CO2) and light alkanes (CH4, C2H6, etc.) in the effluent of the reactor were monitored by an online Agilent Micro GC3000A gas chromatograph (GC) equipped with the molecular sieve, Plot-Q and Al2O3 capillary columns and a TCD detector. The oil and wax products were separated using a cold trap (271 K) and a hot trap (423 K), respectively, while the aqueous products were obtained by phase separation in those traps. The oil products were analyzed using an Agilent 6890N GC with a FID detector and a HP-5 column; the wax products were dissolved in CS2 and analyzed using an Agilent 7890A GC equipped with a FID detector and a HT5 column; the aqueous fraction was analyzed using an Agilent 4890 GC equipped with a FID detector and a HP-Innowax column. The product selectivity was calculated based on the carbon balance.
Li, Z. X., Li, C. S., Ren, J. Y., Li, B. Z. & Zhang, J. Effect of cold deformation on the microstructure and impact toughness during the austenitizing process of 1.0C bearing steel. Mater. Sci. Eng. A. 674, 262–269 (2016).
Cheng, K. et al. Advances in catalysis for syngas conversion to hydrocarbons. Adv. Catal. 60, 125–208 (2017).
In summary, we synthesized graphene layers encapsulated ε-Fe2C nanocrystals for the FT reaction. It exhibited remarkably activity (~ 1258 μmolCO gFe−1 s−1) and stability (>400 h) under realistic FT synthesis conditions. The confinement effects of graphene layers stabilize the metastable but very active ε-Fe2C phase. The unique confinement structure (ε-Fe2C@graphene) can inhibit the formation of an amorphous carbon layer that converts catalytically active ε-Fe2C phase to other less active carbide phases (e.g., χ-Fe5C2). Our results and conclusion could help in the rational design of promising active phases in industrial catalysts for hydrogenation processes.
(a) AFM image of the nano-indentation for the specimen with EPT at peak load of 2 mN; (b) Average hardness of the ferrite matrix for the CR specimens without EPT and with EPT. The insert is load-displacement curves at peak loads of 2 mN.
Duong, D. L. et al. Probing graphene grain boundaries with optical microscopy. Nature 490, 235–239 (2012).
The effects of a graphene layer on the surface stability and carburization feasibility of ε-Fe2C phases were investigated by ab initio atomic thermodynamics based on DFT calculations. The relative chemical potential of carbon (ΔμC) is relevant in describing the thermodynamics of iron carbides, which can be determined from the equilibrium of carburization reactions of different gas atmospheres for pretreatment or FT reaction at some temperatures and pressures. Higher temperature and lower pressure, as well as higher H2/CO ratio, result in lower ΔμC value indicating a lower carburization ability (Fig. 5a, b). Based on ΔμC, the carbon absorption energy (Eabs) can be derived for describing the carburization reaction from metallic Fe to iron carbide. The volume-normalized carbon absorption energy (Ωabs) was applied to the bulk iron carbides20.
Hong, S. Y. et al. A new synthesis of carbon encapsulated Fe5C2 nanoparticles for high-temperature Fischer-Tropsch synthesis. Nanoscale 7, 16616–16620 (2015).
The development of efficient catalysts for Fischer–Tropsch (FT) synthesis, a core reaction in the utilization of non-petroleum carbon resources to supply energy and chemicals, has attracted much recent attention. ε-Iron carbide (ε-Fe2C) was proposed as the most active iron phase for FT synthesis, but this phase is generally unstable under realistic FT reaction conditions (> 523 K). Here, we succeed in stabilizing pure-phase ε-Fe2C nanocrystals by confining them into graphene layers and obtain an iron-time yield of 1258 μmolCO gFe−1s−1 under realistic FT synthesis conditions, one order of magnitude higher than that of the conventional carbon-supported Fe catalyst. The ε-Fe2C@graphene catalyst is stable at least for 400 h under high-temperature conditions. Density functional theory (DFT) calculations reveal the feasible formation of ε-Fe2C by carburization of α-Fe precursor through interfacial interactions of ε-Fe2C@graphene. This work provides a promising strategy to design highly active and stable Fe-based FT catalysts.
Our confined iron carbide catalyst was synthesized by a pyrolysis method, followed by reduction with H2 and carburization in syngas flow. X-ray diffraction (XRD) measurements and 57Fe Mössbauer spectroscopy showed that θ-Fe3C was the major Fe phase in the precursor obtained after pyrolysis (Fig. 2a, b). Transmission electron microscopy (TEM) results clarified that most of the iron carbide nanoparticles were well dispersed with a near-spherical morphology and had a mean size of 13.6 nm (Supplementary Fig. 1a). The high-resolution TEM (HRTEM) studies revealed that the θ-Fe3C nanoparticles were surrounded and closely attached by graphene layers (Fig. 2c and Supplementary Fig. 1b). The lattice fringes with d spacing values of 0.30 and 0.34 nm, which corresponded to the (111) facet of θ-Fe3C and the (002) facet of graphene, respectively, were observed (the insert of Supplementary Fig. 1b). These results indicate that the precursor obtained after pyrolysis is in the structure of θ-Fe3C@graphene.
As a key shape-forming procedure prior to heat treatment, the cold rolling (CR) technology has been proven to have great potential in improving the strength and toughness of low-alloyed bearing steel owing to grain refinement2,3, martensite refinement4, bainite refinement5,6 and solution strengthening7. This raises the speculation that whether the application of CR process will be positive to the comprehensive performance of high-alloyed M50 bearing steel as well. Unfortunately, some results8,9 have shown that the mismatch of strain between the hard phases (cementite or alloy carbides) and soft phases (ferrite) could result in the opening up of voids at the carbides/ferrite interfaces during the CR process with a large plastic deformation, which will deteriorate the final mechanical properties of bearing steel. For the high-alloyed M50 steel with poor formability, the voids are more likely to form during the CR process10. As a result, the industrial application of CR technology in M50 bearing steel has been limited due to the difficulty in preventing or eliminating the nano-size voids.
Garg, A., McNelley, T. R. & Perry, J. L. Analysis of microporosity associated with insoluble carbides in VIM-VAR AISI M-50 steel. Metallography. 20, 89–98 (1987).
Torres Galvis, H. M. et al. Iron particle size effects for direct production of lower olefins from synthesis gas. J. Am. Chem. Soc. 134, 16207–16215 (2012).
The CO chemisorption was performed using a Micromeritics AutoChem II 2920 unit. Before CO chemisorption, 0.1 g of catalyst was reduced under flowing pure hydrogen at 623 K for 3 h and then carburized in syngas (H2/CO = 1) at 573 K for 5 h. Subsequently, the adsorbed species were removed by flowing He at 823 K for 2 h. The samples were cooled to 308 K, then CO chemisorption experiment was conducted. The average CO:Fe stoichiometry was assumed 1:2. For θ-Fe3C@graphene catalyst, reduction and carburization processes are eliminated.
M50 bearing steel used in this study with the nominal composition is presented in Table 1. The material was received as spheroidize-annealed bar and an initial microstructure of primary carbides in ferritic matrix. The ring blank used for CR was prepared with the dimension (outer diameter: 54.5 mm; inner diameter: 34.5 mm) and then cold rolled for a total thickness reduction of 50% using a radial ring rolling machine. After the CR tests, the EPT specimens with a size of 16 × 8 × 4 mm were obtained from the CR ring using wire-electrode cutting. The EPT experiments were conducted by a self-made electropulse generator under ambient conditions. The pulsed electric current was applied for a total duration of 160 ms and had a peak current density of 10.7 kA/cm2 with a frequency of 50 Hz. During the EPT, the maximum temperature rising of the specimens caused by joule heating was measured to be 532.0 °C by means of an infrared camera (Fotric 226).
Fischer–Tropsch (FT) synthesis transforms syngas (a mixture of CO and H2) into multi-carbon hydrocarbons, which can be liquid fuels and chemicals. Because FT synthesis is a core reaction in the utilization of various non-petroleum carbon sources (such as coal, natural or shale gas, biomass, and CO2) to supply energy and chemicals, the development of efficient FT catalysts has received much-renewed interest in recent years1,2,3,4,5,6. Iron-based catalysts have widely been used in the industrial FT process because of the low cost of iron, wide operation conditions, and flexible product distributions7. However, Fe-based catalysts usually suffer from low activity and stability3, and thus many recent fundamental studies have been devoted to enhancing the FT activity and stability of Fe catalysts by employing different modifiers or different supports8,9,10,11,12,13,14. Unlike Ru- or Co-based FT catalysts, where metallic Ru0 or Co0 functions as the active phase, metallic Fe0 is unstable and the evolution of a conventional Fe-based catalyst typically results in a mixture of different iron phases including Fe3O4 and iron carbides under FT reaction conditions15,16,17,18,19,20,21. Iron carbides are believed to be responsible for the activation of CO and the chain growth in FT synthesis, but the nature of the true active iron-carbide phase is still under debate and this hinders the rational design of highly active and stable Fe-based FT catalysts.
After the reduction by H2 at 623 K, α-Fe became the major iron phase, indicating that θ-Fe3C was decomposed into Fe (Fig. 2a). This was also confirmed from HRTEM images, showing that the nanoparticle of α-Fe remains encapsulated by graphene layers after the reduction. The carburization of α-Fe@graphene under syngas at 573 K transformed α-Fe into iron carbide again (denoted as ε-Fe2C@graphene-C). It is of interest that ε-iron carbide (ε-Fe2C) rather than θ-Fe3C or χ-Fe5C2 was formed after the carburization (Fig. 2a). The HRTEM result reveals that the ε-iron carbide is surrounded by the graphene layers (Fig. 2d). It can be clearly seen by spherical aberration-corrected scanning transmission electron microscopes (Cs-corrected STEM) that the graphene layers of ε-Fe2C@graphene catalyst are about 2–7 layers and a large number of defects spread over carbon layer outside ε-Fe2C@graphene catalyst (Supplementary Fig. 2).
a Conventional catalysts with unconfined iron carbide (FexC) particles as the active phase. b Graphene layer-confined ε-Fe2C.
Fu, Q. & Bao, X. Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 46, 1842–1874 (2017).
Quasi in-situ observation of the microstructure for the CR specimens (a) before and (b) after EPT; EDS results of (c) point A and (d) point B.
Raman spectroscopy was used to identify the nature of the graphene layers. Two bands at 1350 and 1590 cm−1 were observed in the Raman spectra for ε-Fe2C@graphene-C and ε-Fe2C@graphene-S, corresponding to the D-band (disordered carbon) and the G-band (graphene carbon), respectively (Supplementary Fig. 5). A lower ID/IG intensity ratio for the ε-Fe2C@graphene was observed, consistent with a higher degree of graphene of the carbon matrix35. The 2D bands of the two ε-Fe2C@graphene samples are closely related to the band structure of graphene layers36. These observations suggest that while the iron phase undergoes changes under different atmospheres, the graphene layers keep surrounding the particle of iron or iron carbide.
Yang, C. L., Yang, H. J., Zhang, Z. J. & Zhang, Z. F. Recovery of tensile properties of twinning-induced plasticity steel via electropulsing induced void healing. Scr. Mater. 147, 88–92 (2018).
Puga, A. V. On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catal. Sci. Technol. 8, 5681–5707 (2018).
Xu, K. et al. ε-Iron carbide as a low-temperature Fischer-Tropsch synthesis catalyst. Nat. Commun. 5, 5783–5783 (2014).
Tsuji, N. & Maki, T. Enhanced structural refinement by combining phase transformation and plastic deformation in steels. Scr. Mater. 60, 1044–1049 (2009).
In order to characterize the distribution of voids in the matrix before and after EPT, the CR specimens without and with EPT were micro-machined and detected by a Zeiss Auriga field emission scanning electron microscopy (FESEM) equipped with a focused ion beam (FIB) and an energy dispersive spectroscopy (EDS). Based on at least eight FIB-SEM micrographs, the distribution of equivalent diameter of voids was measured using Photoshop and Image-pro Plus software. The quasi in-situ observation of the voids was performed in the same field through marking with hardness pits. To investigate the size of carbide particles, the carbides in specimens were extracted by chemical dissolution of the matrix in a modified Berzelius solution15 and then analyzed by a Malvern nano particle size analyzer. Additionally, an FEI tecnai F20 transmission electron microscopy (TEM) was employed to observe the microstructure before and after EPT. The specimens for TEM were prepared by mechanically polishing and then electro-polishing in a twin-jet polisher using a solution of 10% perchloric acid and 90% acetic acid.
Zhou, Y. Z., Zeng, Y., He, G. H. & Zhou, B. L. The healing of quenched crack in 1045 steel under electropulsing. J. Mater. Res. 16, 17–19 (2001).
Das, A., Ghosh, M., Tarafder, S., Sivaprasad, S. & Chakrabarti, D. Micromechanisms of deformation in dual phase steels at high strain rates. Mater. Sci. Eng. A. 680, 249–258 (2017).
Ben, D. D. et al. Declined fatigue crack propagation rate of a high‐strength steel by electropulsing treatment. Adv. Eng. Mater. 1801345, 1–8 (2019).
Lu, X. H., Qian, D. S., Li, W. & Jin, X. J. Enhanced toughness of bearing steel by combining prior cold deformation with martensite pre-quenching and bainite transformation. Mater. Lett. 234, 5–8 (2019).
Bridge, J. E., Maniar, G. N. & Philip, T. V. Carbides in M-50 high speed steel. Metall. Trans. 2, 2209–2214 (1971).
Ni, Z., Wang, Y., Yu, T. & Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 1, 273–291 (2008).
Budrugeac, P. & Segal, E. Applicability of the Kissinger equation in thermal analysis. J Therm Anal Calorim. 88, 703–707 (2007).
Here, we develop a surface-normalized carbon absorption energy (ωabs), which is suitable for describing the surface carburization of iron carbide with and without graphene confinement. The most abundant (101), (1-21), and (2-21) surfaces were selected to evaluate the confinement effects of graphene layers on the ωabs (Fig. 5c). The negative ωabs values indicate the favorable stability of the ε-Fe2C surfaces under the conditions of pretreatment and FT reaction. Although the surface stability follows the order of (101) > (1-21) > (2-21) according to the calculated surface energy (Supplementary Table 4), the (1-21) has the highest carburization feasibility from metallic iron because of the lowest ωabs value.
Ma, Y. R., Yang, H. J., Tian, Y. Z., Pang, J. C. & Zhang, Z. F. Hardening and softening mechanisms in a nano-lamellar austenitic steel induced by electropulsing treatment. Mater. Sci. Eng. A. 713, 145–150 (2018).
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Density functional theory calculations were performed using the generalized gradient approximation Perdew–Burke– Ernzerhof of (PBE) functional44 and projector-augmented wave (PAW) method45 as implemented in the Vienna ab initio simulation package (VASP)46. A second-order Methfessel–Paxton electron smearing scheme (sigma = 0.2 eV) was used because of the metallic conductor properties of iron carbide. Plane-wave kinetic energy cut off of 400 eV is sufficiently accurate for the spin polarization calculations of the electronic properties of open-shell iron carbide. Energy and force convergence criteria were 10−5 eV and 0.03 eV Å−1, respectively. The most abundant ε-Fe2C(1-21), ε-Fe2C(101), and ε-Fe2C(2-21) surfaces were taken into account. A vacuum of 15 Å was used for screening the interactions vertical to the surface. The most stable configurations were selected to model the confinement effects of graphene and N-doped graphene (graphene-N) on the carburization feasibility of ε-Fe2C from metallic iron and the reaction mechanism of CO dissociation. The computational details are deposited in Supplementary Methods.
Figure 4 shows the TEM observation of the microstructure for the CR specimens without and with EPT. For the CR specimens without EPT, the void adjacent to carbide can be found as shown in Fig. 4(a). The HRTEM observation in Fig. 4(b,c) indicates that high dislocation tangle has entangled in the ferrite matrix around carbides for the CR specimens. This result may lie in the fact that the carbides could act as the strong obstacles to inhibit dislocation motion during the CR process. However, the significant recovery of entangled dislocations occurs within a short time of EPT (Fig. 4(d)), which could be attributed to the increased mobility of dislocation by the effect of electron wind force17. In addition, it is also showed that fine recrystallization grains formed around the carbide, indicating the occurrence of rapid recrystallization during the EPT.
Time-on-steam evolution of CO conversion for ε-Fe2C and χ-Fe5C2 at the same conversion (~ 50%) with different GHSVs revealed that the ε-Fe2C@graphene is much more active than χ-Fe5C2 in FT synthesis, as shown in Supplementary Fig. 8. It was also found that the carburization of Fe@graphene into ε-Fe2C@graphene was completed during 50 h at GHSV of 64.0 L gcat−1 h−1. For evaluation of catalyst deactivation, we have performed long-termed FT reactions for both ε-Fe2C@graphene and un-encapsulated χ-Fe5C2 catalysts at 573 K under harsh conditions (Fig. 4b). The CO conversion underwent a significant decrease when the time-on-steam exceeded 100 h for the un-encapsulated χ-Fe5C2 catalyst and this catalyst was covered by carbon. The pressure drop across the un-encapsulated χ-Fe5C2 catalyst bed increased after 100 h and the gas flow was totally blocked at 160 h, as shown in Fig. 4b. On the other hand, the encapsulated ε-Fe2C sample was stable for more than 400 h even at a higher CO conversion (~ 95%). Further, the pressure drop across the ε-Fe2C@graphene catalyst bed was negligible even at high conversion, indicating that the coke deposition on this catalyst was significantly suppressed by the graphene layers under harsh condition.
An, B., Cheng, K., Wang, C., Wang, Y. & Lin, W. Pyrolysis of metal-organic frameworks to Fe3O4@Fe5C2 core-shell nanoparticles for Fischer-Tropsch synthesis. ACS Catal. 6, 3610–3618 (2016).
Khodakov, A. Y., Chu, W. & Fongarland, P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 107, 1692–1744 (2007).
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Zhou, W. et al. New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 48, 3193–3228 (2019).
In addition, the surface composition of the graphene layers is of particular interest and surface contents of N, O, and Fe were determined by X-ray photoelectron spectroscopy (XPS). The θ-Fe3C@graphene sample has a surface atomic ration of Fe/C of about 0.03, and the surface contents of N, O, and Fe elements did not vary with an increase of the Fe loading in the catalysts (Supplementary Table 2). The results revealed a negligible surface iron content, suggesting that θ-Fe3C nanoparticles were encapsulated by the graphene layers in the catalysts.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
In general, the void healing effect induced by EPT can be explained by the inhomogeneous temperature rise caused by the difference of electric resistance between voids and matrix. This inhomogeneous temperature rise can lead to inhomogeneous thermal expansion, so that the voids will be subjected to a strong thermal compressive stress and then consequently healed19. In this work, for the tip region of deformed carbide, the temperature rise around the carbide will be higher because of the detour effect of EPT. As a result, the thermal compressive stress will be greater around the tip region of carbides, which may be more conducive to the healing of the voids around the deformed carbides. Additionally, the dislocation motion around carbides may be another important factor to facilitate the voids healing process. The drift electrons can exert a push on dislocations when high density electric pulses are passing through the CR specimen. Under this force, the rearrangement and annihilation of the dislocations around carbides (Fig. 4(d)) will increase the diffusion velocity of atoms20. Meanwhile, it has been experimentally proved that the voids healing process is controlled by lattice diffusion (transportation of atoms and vacancies)21. Consequently, the generation of thermal compressive stress and acceleration of atoms diffusion will together contribute to the voids healing process of cold rolled M50 bearing steel during EPT.
a θ-Fe3C@graphene samples reduction under flowing H2 at 623 K for 3 h, and then treated under syngas (H2/CO = 1) at different temperature. b θ-Fe3C@graphene samples reduction under flowing H2 at 623 K for 3 h, and then treated under different CO pressure at 573 K.
Niemantsverdriet, J. W. & van der Kraan, A. M. On the time-dependent behavior of iron catalysts in Fischer-Tropsch synthesis. J. Catal. 72, 385–388 (1981).
Ryttberg, K. et al. The effect of cold ring rolling on the evolution of microstructure and texture in 100Cr6 steel. Mater. Sci. Eng. A. 527, 2431–2436 (2010).
We acknowledge Dr. Kenneth G. Rappé (Pacific Northwest National Laboratory), Prof. Norbert Kruse (Washington State University), Prof. Zhao-Xu Chen (Nanjing University), and Prof. Jianyu Huang (Yanshan University) for helpful discussions. This work was supported by the National Natural Science Foundation of China (22072184, 21972170), the Joint Fund of National Natural Science Foundation of China (U1463210).
Lu, J. et al. Promotion effects of nitrogen doping into carbon nanotubes on supported iron Fischer-Tropsch catalysts for lower olefins. ACS Catal. 4, 613–621 (2014).
Cheng, J. et al. Density functional theory study of iron and cobalt carbides for Fischer-Tropsch synthesis. J. Phys. Chem. C. 114, 1085–1093 (2010). M.
L.W. and J.L. supervised the whole project and designed the study. L.W., J.L., and Y.W. co-wrote the manuscript. S. L., S.Y., and J.C. performed most of the experiments and analyzed the experimental data. Z.L. helped to do the DFT simulations. Y.Z. performed reaction testing. All the authors discussed the results and assisted during the manuscript preparation.
Feng Wang, Dongsheng Qian and Lin Hua conceived and designed the experiments; Feng Wang and Lechun Xie performed the experiments and contributed analysis tools; Huajie Mao and Lechun Xie analysed the data; Feng Wang wrote the paper and made all the figures and tables in this paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
de Smit, E. et al. On the surface chemistry of iron oxides in reactive gas atmospheres. Angew. Chem. Int. Ed. 50, 1584–1588 (2011).
Zhao, S. et al. Surface morphology of Hägg iron carbide (χ-Fe5C2) from ab initio atomistic thermodynamics. J. Catal. 294, 47–53 (2012).
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon request.
de Smit, E. & Weckhuysen, B. M. The renaissance of iron-based Fischer-Tropsch synthesis: on the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 37, 2758–2781 (2008).
Figure 4(a) shows that high density dislocation is adjacent to a ferrite/carbide interface, which are likely to draw carbon atoms from the carbide due to the higher binding energy of carbon atoms with dislocation27. Once the sink of carbon atoms in the ferrite become supersaturated, partial dissolution of carbides will take place. This phenomenon generally occurs during the process of strain aging28,29. However, when kinetic diffusion of carbon atoms is accelerated by the pulsed electric, the localized carbide dissolution would occur within a short time. Meanwhile, it should be noted that the detour effect30 of electric current results in a higher joule heating adjacent to voids and the tip region of carbide than the overall estimated temperature (532 °C), which will lead the local temperature of carbides to be close to the thermodynamic dissolution temperature. Since the thermodynamic dissolution temperature of Cr-rich carbides is lowest23, the partial dissolution and refinement could be easier to achieve. Conclusively, according to the chemical composition and morphology observation of the refined carbides, it can be inferred that the Cr-rich carbide with a higher surface to volume ratio could be partially dissolved and refined by EPT (especially at the tip region of the deformed Cr-rich carbide).
X-ray photoelectron spectroscopy (XPS) measurements were conducted on a VG Multilab 2000 photoelectron spectrometer using Al Kα radiation operated under vacuum (2 × 10−6 Pa) with the binding energy (BE) calibrated using the C 1s peak at 284.6 eV.
FIB-SEM micrographs collected from the CR specimens (a) without and (b) with EPT. Insert is the EDS analysis of the nano-size particles (marked with blue circle). (c) Distribution of equivalent diameter of voids.
This work was financially supported by National Natural Science Foundation of China (51575414, 51875426), Innovative Research Team Development Program of Ministry of Education of China (No. IRT13087) and 111 Project (B17034).
Song, M., Du, K., Wen, S. P., Nie, Z. R. & Ye, H. Q. In situ electron microscopy investigation of void healing in an Al–Mg–Er alloy at a low temperature. Acta. Mater. 69, 236–245 (2014).
Huang, F. et al. Anchoring Cu1 species over nanodiamond-graphene for semi-hydrogenation of acetylene. Nat. Commun. 10, 1–7 (2019).
Therefore, this study presents the effect of EPT on the nano-size voids induced by the CR process, and then the relevant microstructure evolution was characterized and discussed in detail. It is interesting to find that not only the nano-size voids around carbides have been healed extensively, but also the carbides have been refined by the EPT technology.

Figure 1 presents the FIB-SEM micrographs of the CR specimens without and with EPT treatment. As indicated in Fig. 1(a), a large number of voids (marked with yellow arrows) less than 1μm have been introduced after the CR process with a 50% thickness reduction. The microstructure of specimens after EPT is shown in Fig. 1(b), where it can be found that some voids have healed (as marked with green arrows) and the number of voids in the matrix have decreased remarkably after EPT. Meanwhile, the distribution of equivalent diameter of voids is further measured as shown in Fig. 1(c). It can be clearly seen that the diameter of voids significantly decreases after EPT, which thus indicates a clear healing effect. In addition, as marked with blue arrows in Fig. 1(a,b), a substantial number of fine carbide particles can be found in the matrix after EPT, while the carbide sizes in the CR specimens are larger than those in the treated specimens. By means of EDS analysis inserted in Fig. 1(b), the nano-size particle (marked with blue circle) is identified to be Cr-rich carbide.
Bao, J., Yang, G., Yoneyama, Y. & Tsubaki, N. Significant advances in C1 catalysis: highly efficient catalysts and catalytic reactions. ACS Catal. 9, 3026–3053 (2019).
Xu, J. & Bartholomew, C. R. Temperature-programmed hydrogenation (TPH) and in situ Mössbauer spectroscopy studies of carbonaceous species on silica-supported iron Fischer-Tropsch catalysts. J. Phys. Chem. B 109, 2392–2403 (2005).
Liu, X. et al. Environmental transmission electron microscopy (ETEM) studies of single iron nanoparticle carburization in synthesis gas. ACS Catal. 7, 4867–4875 (2017).
Yu, T., Deng, D. W., Wang, G. & Zhang, H. C. Crack healing in SUS304 stainless steel by electropulsing treatment. J. Clean. Prod. 113, 989–994 (2016).
On the other hand, the confinement effects are modeled by covering the ε-Fe2C surfaces with a single graphene layer. The optimized distance between ε-Fe2C and graphene is ca. 3.9–4.3 Å (Fig. 5c) close to the dynamic diameter of reactants and single-chain hydrocarbons42,43, which can afford the catalytic FT reaction on the ε-Fe2C surfaces with the graphene confinements. The ωabs values for the graphene-covered ε-Fe2C are lower than those of the pristine ε-Fe2C surfaces, indicating the improved thermodynamic stability. The N-doped graphene (graphene-N) show similar results to those of graphene.
For graphene layers covered metal catalysts, it was demonstrated that defects on graphene layers render the channels for the diffusion of active species through the grain boundaries on the metal surface33. Molecules such as CO, H2, and H2O can go through domain boundaries and point defects (such as pentagon-heptagon defects and vacancies) on the 2D material overlayers, which mainly follow the defect-aided intercalation mechanism34. In addition, the Raman spectra of ε-Fe2C@graphene catalyst show that the 2D peak position of ε-Fe2C@graphene blueshifted relative to single-layer graphene and the peak pattern consistent to the few graphene layers rather than bulk graphite (Supplementary Fig. 3).
Niemantsverdriet, J. W., Van der Kraan, A. M., Van Dijk, W. L. & Van der Baan, H. S. Behavior of metallic iron catalysts during Fischer-Tropsch synthesis studied with Mössbauer spectroscopy, X-ray diffraction, carbon content determination, and reaction kinetic measurements. J. Phys. Chem. 84, 3363–3370 (1980).
a XRD diffraction patterns for θ-Fe3C@graphene sample (black line), θ-Fe3C@graphene sample reduction under flowing H2 at 623 K for 3 h (red line), and then carbonization under flowing syngas (H2/CO = 1) at 573 K for 10 h (blue line). b 57Fe Mössbauer spectra for θ-Fe3C@graphene, ε-Fe2C@graphene-C, and ε-Fe2C@graphene-S samples. Representative high-resolution TEM micrographs for c θ-Fe3C@graphene and d ε-Fe2C@graphene-C. Scale bar, 2 nm.
The change of free energy (\({\rm{\Delta }}{{G}_{dis}}^{EPT}\)) for the second-phase dissolution process under EPT can be simplified as25:
Kang, S. et al. High-performance Fe5C2@CMK-3 nanocatalyst for selective and high-yield production of gasoline-range hydrocarbons. J. Catal. 349, 66–74 (2017).
Xiong, H. et al. Fischer-Tropsch synthesis: Iron catalysts supported on N-doped carbon spheres prepared by chemical vapor deposition and hydrothermal approaches. J. Catal. 311, 80–87 (2014).
Key Laboratory of Catalysis and Energy Materials Chemistry of Ministry of Education & Hubei Key Laboratory of Catalysis and Materials Science, South-Central University for Nationalities, Wuhan, 430074, China
Santos, V. P. et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 6, 6451–6458 (2015).
State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, National Engineering Laboratory for Green Chemical Productions of Alcohols, Ethers and Esters, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China
Furthermore, the selectivity toward olefins and long-chain hydrocarbons also did not undergo significant changes during the long-term reaction. The hydrocarbon distribution over the ε-Fe2C@graphene catalyst follows the Anderson-Schulz-Flory distribution (Supplementary Fig. 9). Our Raman studies for the catalyst after reaction showed that the graphene layers and ε-Fe2C phase did not undergo significant changes (Supplementary Fig. 5). The morphology and crystalline structure of the ε-Fe2C@graphene catalyst also kept almost unchanged after 400 h reaction.
Xie, J. et al. Size and promoter effects on stability of carbon-nanofiber-supported iron-based Fischer-Tropsch catalysts. ACS Catal. 6, 3147–3157 (2016).
The 57Fe Mössbauer measurements were performed at room temperature or 77 K using a conventional spectrometer (Germany, Wissel MS-500) in transmission geometry with constant acceleration mode. A 57 Co(Rh) source with an activity of 25 mCi was used. The velocity calibration was done with a room temperature α-Fe absorber. The spectra were fitted by the software Recoil using Lorentzian Multiplet Analysis. The samples were passivated in flowing 1% O2/N2 for 1 h at room temperature prior to air exposure and being sealed in a sample holder with paraffin wax for Mössbauer spectroscopy measurements.
(a) The XRD patterns of the CR specimens without and with EPT; the DSC curves of the CR samples (b) without and (c) with EPT at different heating rates; (d) The volume fraction of austenite formed as a function of temperature at 5 °C/min.
Chakraborty, J., Bhattacharjee, D. & Manna, I. Development of ultrafine bainite + martensite duplex microstructure in SAE 52100 bearing steel by prior cold deformation. Scr. Mater. 61, 604–607 (2009).
Thermogravimetric analysis (TGA) was carried out on a NETZSCH TG 209F3 TGA analyzer during temperature ramping from 303 K to 1,173 K in flowing air (50 mL min−1) with a ramping rate of 10 K min−1.
For CO temperature-programmed desorption (CO-TPD), 0.1 g of catalyst was reduced under flowing pure hydrogen at 623 K for 3 h and then carburized in syngas (H2/CO = 1) at 573 K for 5 h. Subsequently, the adsorbed species were removed by flowing He at 573 K for 2 h. The samples were cooled to 308 K. At this temperature, the carburized sample was flushed with CO for 1 h and consequently purged with He until the baseline of CO signal leveled off. Finally, the sample was heated to 1,073 K at a ramp of 10 K min−1. For θ-Fe3C@graphene catalyst, reduction and carburization processes are eliminated.
Wang, F., Qian, D., Hua, L. et al. Voids healing and carbide refinement of cold rolled M50 bearing steel by electropulsing treatment. Sci Rep 9, 11315 (2019). https://doi.org/10.1038/s41598-019-47919-6
a Comparison of FTY values between ε-Fe2C@graphene catalyst and iron catalyst loaded on active carbon with different iron loadings. b Long-term stability of ε-Fe2C@graphene and un-encapsulated χ-Fe5C2 catalysts. Reaction conditions: H2/CO = 1/1, 573 K, p = 10 bar. The insert shows the high-resolution TEM micrograph for the spent ε-Fe2C@graphene catalysts after 400 h reaction. Scale bar, 5 nm.
Liu, X. B. & Zhang, X. F. An ultrafast performance regeneration of aged stainless steel by pulsed electric current. Scr. Mater. 153, 86–89 (2018).
Lyu, S. et al. Role of active phase in Fischer-Tropsch synthesis: experimental evidence of CO activation over single-phase cobalt catalysts. ACS Catal. 8, 7787–7798 (2018).
Jin, Y. & Datye, A. K. Phase transformations in iron Fischer-Tropsch catalysts during temperature-programmed reduction. J. Catal. 196, 8–17 (2000).
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
The iron carbide nanocomposites were synthesized by pyrolysis of a molten mixture of urea, glucose, and Fe(NO3)3•9H2O. In a typical synthesis, an amount of Fe(NO3)3•9H2O, corresponding to the final Fe loading of 30–60 wt%, was mixed with 5.0 g urea and 3.0 g glucose at 393–433 K to form a transparent solution. The resultant molten mixture was heated at 453 K in an oven for 24 h. The solid collected was subjected to a heat treatment in flowing N2 (10 mL min−1) at 673 K for 30 min and finally at the final temperatures (773–1023 K) for another 2 h.
For comparison, we further studied the changes in structures of θ-Fe3C@graphene samples before and after treatment under different conditions (Supplementary Fig. 4). The direct carburization of θ-Fe3C@graphene did not induce any detectable change in the phase composition, probably because this sample was formed by a prior carburization process. Fe2O3 was obtained by oxidation of θ-Fe3C@graphene and served as a carbon encapsulation-free reference. The carburization treatment of Fe2O3 led to the formation of Fe3O4 and χ-Fe5C2. The characterization result shows that only χ-Fe5C2 could be observed at 573 K, which is the thermodynamic stable phase at high temperatures. On the other hand, the carbon-encapsulated metal Fe sample only formed the ε-Fe2C phase after the carburization treatment. The ε-Fe2C phase can be stabilized by the graphene layer even at such a high temperature.
The ε-Fe2C@graphene catalysts with different Fe loadings all showed very high activities. The FTY value for the ε-Fe2C@graphene catalysts with Fe loadings in a range of 10–50 wt% is almost the same (~ 600 µmolCO gFe−1 s−1) under the same reaction condition. On the other hand, the FTY value of reference Fe/C catalysts decreased sharply upon increasing Fe loading probably due to the aggregation of Fe species and the oxidation of the active carbide phase under reaction conditions (Fig. 4a)40,41. The keeping of high FTY value at high Fe loading suggests that the high dispersion of ε-Fe2C phase in the ε-Fe2C@graphene catalyst is sustained at high Fe loadings and the ε-Fe2C keeps stable during FT reaction. The FTY value of ε-Fe2C@graphene with iron loading of 40.5 wt% reached 1258 μmolCO gFe−1 s−1 when the gas-hour space velocity (GHSV) was increased to 160 L gcat−1 h−1 at 613 K (Fig. 4a). The FTY values reported to date are limited at 1000 μmolCO gFe−1 s−1 (Supplementary Table 3) and the FTY value obtained using the ε-Fe2C@graphene catalyst breaks this limitation.
Okitsu, Y., Takata, N. & Tsuji, Y. N. A new route to fabricate ultrafine-grained structures in carbon steels without severe plastic deformation. Scr. Mater. 60, 76–7 (2009).
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
The voids caused by the cold rolling (CR) quite deteriorates the final performance of M50 bearing steel. In this work, the effect of electropulsing treatment (EPT) on the voids has been investigated, finding that the nano-size voids around carbides have been extensively healed. Moreover, it is interesting to find that the Cr-rich carbides are partially dissolved and consequently refined by EPT, which could be attributed to the decreased thermodynamic dissolution barriers and accelerated kinetic diffusion of carbon atoms towards dislocation. These results inspire people to develop a novel strategy (CR + EPT) to fully take advantage of CR and tailor the carbides size in bearing steels.
Sheng, Z. H. et al. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 5, 4350–4358 (2011).
Chen, X., Deng, D., Pan, X., Hu, Y. & Bao, X. N-doped graphene as an electron donor of iron catalysts for CO hydrogenation to light olefins. Chem. Commun. 51, 217–220 (2015).
Xu, X. F., Zhao, Y. G., Wang, X. D., Zhang, Y. Y. & Ning, Y. H. Effect of rapid solid-solution induced by electropulsing on the microstructure and mechanical properties in 7075 Al alloy. Mater. Sci. Eng. A. 654, 278–281 (2016).
Here, the thermal stability ε-Fe2C@graphene enables us to investigate its catalytic performance at 573 K. The FTY value of ε-Fe2C@graphene at 573 K was 582.8 μmolCO gFe−1 s−1 (Table 1), which was significantly higher than those for the un-encapsulated χ-Fe5C2 derived from Fe2O3 and the θ-Fe3C@graphene catalysts. The intrinsic activity (TOF values) of ε-Fe2C is ~6–10 times higher than the θ-Fe3C and 2 times higher than the χ-Fe5C2. Furthermore, CO-TPD profiles of ε-Fe2C@graphene catalyst show a multi-peak overlapped cure with a maximum peak position at ca. 843 K, which is attributed to desorption of CO after recombination of dissociated carbon and oxygen on the surface (Supplementary Fig. 7). The result revealed the strongly bound CO on the surface due to the confinement effect of ε-Fe2C@graphene catalyst. In addition, the CO2 selectivity for the ε-Fe2C@graphene catalyst was lower than those for the other two reference catalysts, indicating that the ε-Fe2C is a more active and selective phase for the conversion of syngas to hydrocarbons.
Gouné, M., Bouaziz, O., Pipard, J. M. & Maugis, P. Study of the effect of cold deformation on the austenite formation. Revue de Métallurgie. 103, 465–471 (2006).
Here, we attempt to replace the amorphous carbon with few graphene layers that confines the Fe-based catalyst. The confinement of the rigid geometry of the graphene shell can inhibit the formation of the amorphous carbon layer and improve the stability of highly active ε-Fe2C (Fig. 1b). We report our finding that the confinement of ε-Fe2C inside graphene layers (denoted as ε-Fe2C@graphene) can stabilize this metastable phase for FT synthesis at 523–613 K. The Fe-time yield (FTY), which is defined as the moles of CO converted to hydrocarbons per gram of Fe per second, reaches 1258 μmolCO gFe−1 s−1 at 613 K, breaking the upper-limit value (1000 μmolCO gFe−1 s−1) reported for Fe-based FT catalysts12,13. The catalyst is highly stable under our FT reaction conditions and high CO conversion (~ 95%) can be kept at 573 K at least for 400 h. DFT calculations suggest that the confinement effects of graphene layers favor the formation of ε-Fe2C from carburization of α-Fe, which maintains the high stability of ε-Fe2C under high-temperature FT reaction conditions. On the other hand, the facile transformation of FexC particles may occur during FT synthesis over the conventional FT catalyst. To the best of our knowledge, this is the first example to demonstrate experimentally that the ε-Fe2C phase can be stabilized under high-temperature FT reaction conditions. The present work provides a promising strategy to synthesize highly active and stable Fe-based FT catalysts and offers an opportunity for the study of FT reactions on pure metastable-phase iron carbides.
Kang, M. K. et al. Carbon content of bainite ferrite in 40CrMnSiMoV steel. Mater. Chem. Phys. 118, 438–441 (2009).
Lyu, S., Wang, L., Li, Z. et al. Stabilization of ε-iron carbide as high-temperature catalyst under realistic Fischer–Tropsch synthesis conditions. Nat Commun 11, 6219 (2020). https://doi.org/10.1038/s41467-020-20068-5
de Smit, E. et al. Stability and reactivity of ε-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: controlling μc. J. Am. Chem. Soc. 132, 14928–14941 (2010).
Yang, C., Zhao, H., Hou, Y. & Ma, D. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 134, 15814–15821 (2012).
The size and morphology of samples were determined using a FEI Tecnai G20 transmission electron microscope operated at 200 kV and a Hitachi SU8000 field emission scanning electron microscope at an accelerating voltage of 15 kV.
Sprecher, A. F., Mannan, S. L. & Conrad, H. Overview no. 49: On the mechanisms for the electroplastic effect in metals, Acta. Metall. 34, 1145–1162 (1986).
The novel phenomenon provides a new pathway for fully eliminating the deteriorating effect of voids on mechanical properties and maximizing the advantages of CR in bearing steel. Meanwhile, it is of great engineering significance to tailor the carbides size in bearing steels using the efficient and energy-saving EPT processing technology, in the context of cold rolled bearing steel.
SEM observation of the extracted carbides from the CR specimens (a) without and (b) with EPT, and (c) diameter distribution of extracted carbides.
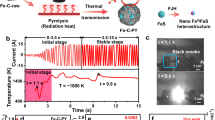
As shown in Fig. 3, the carbides in the CR specimens without and with EPT are extracted and then observed by SEM. It clearly shows that the extracted carbides of the EPT specimen is finer than that of specimen without EPT. According to the analysis of particle size of carbides, the average diameter of carbides decreases from 495 nm to 412 nm after EPT. Furthermore, the particle size distribution of extracted carbides (Fig. 3(c)) indicates that the proportion of carbides with a diameter less than 200 nm is significantly increased after EPT, which therefore coincides with the observation in Fig. 1(b).
Recently, many researches11,12,13,14 have experimentally proven that the macro cracks can be effectively repaired by electropulsing treatment (EPT) due to the temperature rise and compressive stress. Thus, this crack-healing effect gives us a vision that the combination of CR and EPT may further improve the mechanical properties of M50 bearing steel if the nano-size voids around carbides can be healed as well. However, it is worth noting that the voids resulted from CR process mainly locate around high resistance second-phase (carbides), making the application of EPT to affect the nano-size voids more complicated. Besides, the dislocation entanglement induced by CR may also exert a crucial effect on the void healing.

The θ-Fe3C@graphene samples obtained above were reduced in a flow of 3 L gcat−1 h−1 of H2 at 623 K for 3 h (denoted as Fe@graphene), and then the carbonization in a flow of 64 L gcat−1 h−1 of syngas (H2/CO = 1) at 573 K for 10~400 h (denoted as ε-Fe2C@graphene).
Furthermore, the carbon-supersaturated ferrite results in a decrease of the carbon chemical potential between the ferrite and carbides, which will facilitate the transformation of ferrite to austenite33. Figure 6 (b,c) illustrates the DSC curves of the CR samples without and with EPT at different heating rates. Obviously, the peak transformation temperature of α → γ increases with the increase of heating rate. Based on the Kissinger method34, the activation energy for the transformation from ferrite to austenite (Qα→γ) is obtained in Table 2, which indicates a distinct decrease of Qα→γ after EPT. Meanwhile, the volume fraction of austenite formed as a function of temperature at 5 °C/min is plotted in Fig. 6(d), which demonstrates that the transformation of α → γ shifts to lower temperature. Therefore, the EPT not only leads to partial dissolution of carbides but also accelerates the transformation from ferrite to austenite during subsequent heating by increasing the carbon solid-solute in ferrite.
XPS characterizations were further performed to analyze carbon and nitrogen bonding configurations in the carburization process of Fe@graphene. N 1s peaks in XPS of θ-Fe3C@graphene (Supplementary Fig. 6a, b) can be fitted into four peaks at 398.3 eV, 399.6 eV, 400.8 eV, and 402.5 eV referring to the pyridinic, pyrrolic, graphitic and oxidized nitrogen, respectively37. These results confirmed the existence of N functional groups (pyridinic-N, pyrrolic-N, and graphitic-N), indicating unique defect-rich structure graphene layers after the annealing process. Moreover, the XPS results clearly confirmed the presence of defective graphene layers during the reducing and carburization processes (Supplementary Fig. 6c, e) and the incorporation of nitrogen atoms within the graphene layers (Supplementary Fig. 6d, f). From the above Raman spectra, STEM/HRTEM images, and XPS results, we conclude that the highly defective graphene layers have been successfully synthesized during the thermal annealing. The number of defects and type of doped N in carbons might play a crucial role in enhancing FT reaction catalytic performance38,39. N 1s XPS spectra of the few graphene layers confined iron catalysts reveals that the graphitic N is the most abundant N species, indicating that the graphitic N could affect the performance of confined iron catalysts, which is rationalized by our theoretical modeling shown in the part of DFT calculation.
Lee, H. K. et al. Extremely productive iron-carbide nanoparticles on graphene flakes for CO hydrogenation reactions under harsh conditions. J. Catal. 378, 289–297 (2019).
For observing the effect of EPT on the hardness of ferrite matrix adjacent to carbides, the nano-indentation tests were carried out before and after EPT at peak loads of 1, 2 and 3 mN using a nano-indentation system (NH2, Switzerland). The indentation was applied on the ferrite matrix and confirmed by an atomic force microscope (AFM; DI Nanoscope IV). To investigate the crystal structure information of ferrite, the X-ray diffraction (XRD) data were obtained with a scanning speed of 1°/min on a Rigaku D/MAX-RB diffraction analyser at 12 kW. Furthermore, the differential scanning calorimetry (DSC) experiments were also performed using a PerkinElmer Pyris 1 calorimeter. The specimens for DSC were cut into ϕ 4 mm × 0.5 mm and then heated from ambient temperature to 950 °C at different heating rates of 5, 10, 15, 20 °C/min, respectively.
To gain further insights into the possible evolution of iron phases, we have performed in situ XRD characterizations for our sample in syngas flow under different conditions. α-Fe was still the major phase under syngas with a H2/CO ratio of 1 at 473 K and was gradually changed to ε-Fe2C phase upon increasing the temperature from 473 to 573 K (Fig. 3a). In addition, during the carburization process, ε-Fe2C phase kept stable in syngas flow at 573 K for 5 h and remained almost unchanged by changing the CO pressure at 573 K (Fig. 3b). It is noteworthy that conventionally ε-Fe2C is unstable and would be converted to χ-Fe5C2 and θ-Fe3C at ≥ 523 K20. Interestingly, we observed the formation of ε-Fe2C phase in a wide range of temperature and CO pressure, which are closely related to the model of carbon chemical potential (μC) as explained in the computational details of Supplementary Methods. Thus, the present results clearly demonstrate that the confinement of ε-Fe2C inside graphene layers can keep it from phase transformations probably.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In addition, taking (1-21) as an example, we further investigate the mechanism of CO dissociation on the ε-Fe2C surface with and without graphene confinement. Both the direct and H-assisted dissociations are considered including different active sites of perfect ε-Fe2C, graphene, and the C-vacancy on ε-Fe2C (Supplementary Figs. 11–15). The C-vacancy on ε-Fe2C is more active for the direct CO dissociation with a lower energy barrier (Ea) of ca. 1.2 eV than those of direct and H-assisted dissociations (2.57-2.61 and 2.83-2.87 eV) on the perfect ε-Fe2C surface with and without the confinement of graphene. The CO dissociation on the graphene site hardly occurs due to the high Ea values (> 2.89 eV). Similar results can be found for graphene-N. The confinement of graphene or graphene(-N) favors to improve the stability of the highly active ε-Fe2C to achieve the high catalytic performance of FT at high temperature.
Jiang, Y. B., Tang, G. Y., Shek, C. H. & Liu, W. Microstructure and texture evolution of the cold-rolled AZ91 magnesium alloy strip under electropulsing treatment. J. Alloy. Compd. 509, 4308–4313 (2011).
a Relative chemical potential of carbon (ΔμC) for carburization by CO (2CO → C + CO2). b Relative chemical potential of carbon (ΔμC) for carburization by syngas (4CO + 4H2 → 2C + CO2 + 2H2O + CH4). c Surface-normalized carbon absorption energy (ωabs) of ε-Fe2C surfaces with and without graphene(-N) layers and the most stable structures labeled by the distances between ε-Fe2C and graphene (data in parenthesis referring to those of graphene-N).
To further verify the occurrence of voids healing, the quasi in-situ observation of the void is performed as shown in Fig. 2. The initial void with long axis of about 260 nm locates between two strip carbides, which may be caused by the movement of carbide fragments during plastic deformation16. After EPT, the diameter of void decreases to about 80 nm, thereby validating the contribution of EPT to the healing of the voids around carbides. It is also found that the tip region of deformed carbide has been partially disappeared after EPT as circled in Fig. 2(b), which may result from the localized dissolution of carbides. Furthermore, the EDS results of point A and B are shown in Fig. 2(c,d). It can be seen that there is larger amount of Mo element existing in the broken strip carbide (marked with A), whereas the partially dissolved carbide is rich in Cr element (marked with B). The results, together with the chemical analysis in Fig. 1, prove that the Cr-rich carbides have lower stability under EPT.
It was once reported that the activity of ε-Fe2C nanoparticles is 4.3 times that of χ-Fe5C2 and is even comparable to that of the precious metal Ru for FT synthesis, probably owing to its excellent ability to dissociate CO29. Unfortunately, the study of the catalytic performance of ε-Fe2C under practical FT reaction conditions is limited because of its metastable state.
M50 bearing steel has been widely used in the aerospace industry as main shaft bearing in gas-turbine engines due to its excellent elevated temperature performance1. In recent years, with the rapid development of aerospace industry, the demand for high properties of aviation bearing steel is constantly increasing for the sake of adapting to the worse working conditions. Therefore, how to improve the mechanical properties of M50 bearing steel has attracted much attention.
Schulte, H. J., Graf, B., Xia, W. & Muhler, M. Nitrogen- and oxygen- functionalized multiwalled carbon nanotubes used as support in iron-catalyzed, high-temperature Fischer-Tropsch Synthesis. ChemCatChem 4, 350–355 (2012).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Azuma, M. Structural control of void formation in dual phase steels, PhD thesis, Technical university of Denmark, (2013).
Wang, F., Qian, D. S., Hua, L. & Lu, X. H. The effect of prior cold rolling on the carbide dissolution, precipitation and dry wear behaviors of M50 bearing steel. Tribol. Int. 132, 253–264 (2019).
Hägg χ-Fe5C2 has been observed in many Fe-based catalysts after FT reactions or during in situ characterizations22,23,24. These observations form the current consensus that χ-Fe5C2 is the active phase for FT synthesis. The pure-phase χ-Fe5C2 was also successfully synthesized and was confirmed to be efficient for FT synthesis25,26. Theoretical calculations also predicted that χ-Fe5C2 surfaces catalyzed the CO activation and C-C chain growth27,28, and χ-Fe5C2 should be more active than metallic Fe27. Nevertheless, a recent work disclosed that the octahedral carbide ε-Fe2C, which contains carbon atoms in octahedral interstices of hexagonal closed-packed iron lattice, was more active than a χ-Fe5C2-dominant catalyst in the low-temperature (≤473 K) FT reaction29. The Fe catalyst based on ε-Fe2C phase could also decrease the CO2 selectivity during FT synthesis30. However, it is known that ε-Fe2C would be transformed into χ-Fe5C2 at above 523 K, and thus would be unstable at a higher temperature (~573 K) that is usually adopted for Fe-catalyzed FT synthesis. Under FT reaction, Fe-based catalysts are usually coated with an amorphous carbon/carbide layer that facilely induces the carbides transformation (Fig. 1a)31,32. Thus, it is highly challenging to synthesize stable catalysts that are dominated by the highly active ε-Fe2C phase for FT synthesis.
Torres Galvis, H. M. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 335, 835–838 (2012).
Lu, Z. C. et al. Effect of electropulsing treatment on microstructure and mechanical properties of intermetallic Al3Ti alloy. J. Alloy. Compd. 708, 834–843 (2017).
Kemp, I. P., Pollard, G. & Bramley, A. N. Static strain aging in high carbon steel wire. Mater. Sci. Tech. 6, 331–337 (1990).
Cabrol, E., Bellot, C., Lamesle, P., Delagnes, D. & Povoden-Karadeniz, E. Experimental investigation and thermodynamic modeling of molybdenum and vanadium-containing carbide hardened iron-based alloys. J. Alloy. Compd. 556, 203–209 (2013).



 18581906093
18581906093