20830 - 20830
The final optical microstructure appears as in Fig. 11, consisting of colonies of pearlite, i.e., regions which participated in cooperative growth at a common front. In this two-dimensional section, each colony appears as if it is a stack of layers of cementite and ferrite. The colonies appear to have different interlamellar spacing, but this may be a sectioning effect.
The word allotriomorph implies that the shape of the ferrite does not reflect its internal crystalline symmetry. This is because it tends to grow more rapidly along the austenite grain surface and hence its contours refect those of the γ grain boundary. In contrast, an idiomorph is not influenced by the boundary and hence has a crystallographically facetted shape. This is illustrated schematically in Fig. 5 and actual micrographs of an allotriomorph and idiomorph are presented in Figs. 6, 7.
In the midst of the competition, the Tiger 24 required an unexpected engine swap leading to some unforeseen challenges the following day. However, this situation led to new opportunities for learning and improvement, allowing the team to focus on future successes and ultimately strengthen their resilience and determination.
Be sure to follow CUFSAE on Instagram, Facebook, and LinkedIn to stay current on all of the innovative things they are doing.
Additionally, Rowen Smith is the new Sponsorship Coordinator for CUFSAE. With these changes to the team, Smith says that they will aspire to focus on reliability, better teamwork and earlier production for next year’s car, the Tiger 25, which will undergo design this summer.
In some steels containing a strong carbide-forming elements such as Mo or V, tempering at temperatures where these solutes are mobile leads to the precipitation of alloy carbides (Fig. 31).
The following micrographs are courtesy of Arijit Saha Podder. The samples were polished to a mirror finish prior to heat treatment. The thermal grooves reveal the austenite grain boundary structure. The photographs are taken using ordinary reflected light microscopy. Here the sample is transformed to allotriomorphic ferrite to avoid surface relief effects.
Indeed, a colony in three dimensions does not consist of alternating, isolated layers of cementite and ferrite. All of the cementite is a single-crystal, as is all of the ferrite. The colony is therefore an interpenetrating bi-crystal of ferrite and cementite. Imagine in Fig. 12, that the cabbage represents in three dimensions, a single crytal of cementite within an individual colony of pearlite. The leaves of the cabbage are all connected in three dimensions. When the cabbage is immersed in a bucket of water, imagine further that the water is a single crystal of ferrite within the same colony of pearlite. The two will interpenetrate to form the bi-crystal. When this bi-crystal is sectioned, the appearance is that of alternating layers of the two crystals.
Bainitemicrostructure
The following micrographs are courtesy of Arijit Saha Podder. The samples were polished to a mirror finish prior to heat treatment. The thermal grooves reveal the austenite grain boundary structure. The photographs are taken using Nomarsk interference light microscopy. Here the sample is transformed into martensite, so the surface relief effects complicate visualisation of the prior austenite grain boundaries.

Austenite has a cubic-close packed crystal structure, also referred to as a face-centred cubic structure with an atom at each corner and at the centre of each face of the unit cell. Ferrite has a body-centred cubic crystal structure and cementite has an orthorhombic unit cell containing four formula units of Fe3C. The phase diagram illustrates the domains in which particular phases or combinations of phases are stable, and contains information about their equilibrium compositions. Equilibrium phase fractions can also be estimated from a knowledge of the carbon concentration of the steel and an application of the lever rule.
Martensitemicrostructure
An understanding of the atomic mechanisms of solid-state transformation is important because the details of the way in which atoms move determine the morphology, chemical composition and other characteristics of the microstructure.
Clemson Formula SAE (CUFSAE) is a student-led organization at Clemson that is focused on designing, manufacturing and competing with a formula-style race car. Each year, they design and build a new vehicle from the ground up that meets competition rules while also pursuing innovation. May 8-11, CUFSAE competed in the Formula SAE Michigan competition with their newest race car, the Tiger 24.
A civilian transformation is one in which the queue of civilians board the bus in an un-coordinated manner so that all correspondence between the positions in the bus and the queue is lost. Civilians occupy the positions they prefer to occupy, a situation analogous to diffusion.
There is a third kind of transformation, paraequilibrium in which the larger atoms in substitutional sites move in a discipline manner (without diffusion) whereas the faster moving interstitial atoms diffuse and partition between the phases. This is how Widmanstätten ferrite grows, a displacive mechanism whose rate is controlled by the diffusion of carbon in the austenite ahead of the αw/γ interface.
The difference between bainite and martensite is at primarily at the nucleation stage. Martensitic nucleation is diffusionless, but it is thermodynamically necessary for carbon to partition during the nucleation of bainite. Bainite also forms at temperatures where the austenite is mechanically weak. The shape deformation due to the bainite transformation is therefore casues plastic deformation in the adjacent austenite. This deformation stops the bainite plates from growing and transformation then proceeds by the nucleation of further plates, which also grow to a limited size.
It is obvious that the equilibrium phase digram (Fig. 1) does not contain any information about phases such as bainite, martensite etc. This is because it represents equilibrium whereas the variety of transformation products have a range of deviations from the equilibrium state.
Figures 13 and 14 show an optical micrograph and a crystallographic orientation image from the same sample. It is evident from the colour image that the colour (crystallographic orientation) is essentially homogeneous within a colony of pearlite.
In fact, the strain energy due to the shape deformation when an individual plate of Widmanstätten ferrite forms is generally so high that it cannot be tolerated at the low driving-force where it grows. As a consequence, two back-to-back plates which accommodated each others shape deformation grow simultaneously. This dramatically reduces the strain energy, but requires the simultaneous nucleation of appropriate crystallographic variants. As a consequence, the probablity of nucleation is reduces and the microstructure is coarse. The characteristic thin-wedge shape of αw is because the two component plates have different habit plane variants with the parent austenite.
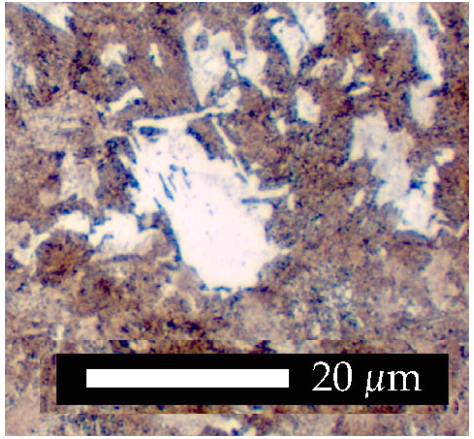
In Fig. 9, the low carbon concentration of the steel allows much more allotriomorphic ferrite to form with the grains therefore appearing equiaxed because of the effects of hard impingment. The amount of pearlite is reduced because of the lower carbon concentration of the steel.
Austenitevs martensite vsferritevspearlite
The austenite grain boundaries are thus destroyed in the process of forming allotriomorphic ferrite or pearlite. This is not the case with displacive transformation products where even if all the austenite is consumed, a vestige of the boundary is left as the prior austenite grain boundary. Austenite grain boundaries and indeed, prior austenite grain boundaries, absorb detrimental impurities. One consequence is that strong steels based on microstructures obtained by displacive transformation become susceptible to impurity embrittlement.
The vast majority of commercial steels contain manganese and are produced by casting under conditions which do not correspond to equilibrium. There are as a result, manganese-enriched regions between the dendrites. Any solid-state processing which involves rolling-deformation is then expected to smear these enriched regions along the rolling direction, thus building into the steel bands of Mn-enriched and Mn-depleted regions.
When the austenite in such steels is cooled, ferrite first forms in the Mn-depleted regions. Ferrite has a very low solubility for carbon which partitions into the Mn-enriched regions which on further cooling, transform into bands of pearlite. The banded microstructure is illustrated in Fig. 20 . Fig. 21 shows microanalysis data which confirm that pearlite tends to form in the Mn-enriched regions.
Steels with a carbon concentration less that the eutectoid marked S on the phase diagram are known as hypoeutectoid and those which exceed this concentration are said to be hypereutectoid.
Pearlitevs martensite
The purpose here is to help identify the microstructures in steel using simple techniques based on the atomic mechanisms by which phases grow from austenite. Apart from their aesthetic beauty, microstructures become meaningful when examined in the context of their metallurgical theory. You can freely download the complete book Theory of Transformations in Steels.
It is sometimes the case that a pearlitic steel is too strong for the purposes of machining or other processing. It can then be heat-treated at a temperature below that at which austenite forms, to allow the cementite to spheroidise. The "lamellae" of cementite turn into approximately spherical particles of cementite in an effort to minimise the amout of θ/α interfacial area/energy per unit volume (Fig. 18).
Images taken by Laura Pocock, of a martensitic weld metal with an extremely fine structure, with individual plates of martensite being about 0.2 micrometres in thickness. These kinds of structures are difficult to index during electron backscattered diffraction. For example, the electron beam spreads within the sample, thereby compromising crystallographic resolution.
Cementitevs martensite
The atomic mechanism of bainite is similar to that of martensite (Fig. 33). Plates of bainite form without any diffusion, but shortly after transformation, the carbon partitions into the residual austenite and precipitates as cementite between the ferrite platelets - this is the structure of upper bainite (Fig. 34). Lower bainite is obtained by transformation at a lower temperature; the carbon partitioning is then slower, so some of the excess carbon has an opportunity to precipitate inside the ferrite plates and the rest of it precipitates from the carbon-enriched austenite as in upper bainite, Fig. 34.
As the extent of transformation increases, the shape of the ferrite will change as grains growing from different origins touch each other (impinge). In Fig. 8, the austenite grain boundaries are completely decorated by ferrite allotriomorphs and the residual austenite has transformed into pearlite (which exhibits typical irridescence).
Transmission electron microscopy can reveal the small amount of inter-plate retained austenite in low-alloy steels (Fig. 29).
Difference betweenbainiteand pearlite
In a hypoeutectoid steel, a colony of pearlite evolves with the nucleation of ferrite as illustrated in Fig. 10. This in turn triggers the nucleation of a particle of cementite and this process repeats periodically. The two phases then are able to establish cooperative growth at the common front with the austenite, with much of the solute diffusion happenning parallel to this front within the austenite. The distance between the "layers" of cementite and ferrite is known as the interlamellar spacing.
A part of the Tiger 24’s success has been credited to the support of CUFSAE’s sponsors. The team has expressed their unwavering gratitude towards their sponsors, including the Robert H. Brooks Sports Science Institute (RHBSSI), saying that the sponsors’ continuous assistance and investment gives the team added strength to keep pushing creativity and build the most competitive car they can. We at RHBSSI commend the CUFSAE team on all their successes to-date and wish them best of luck in their upcoming competitions this season.
Untemperedmartensite
This competition will not be the last chance for the team to display the Tiger 24’s capabilities. They will have the opportunity to further test these abilities in upcoming test days as well as competitions occurring later this year.
Tempering at a low temperature relieves the excess carbon trapped in the martensite, by the precipitation of cementite. The retained austenite is not affected by tempering at temperatures below MS, Fig. 30.
Martensite transformation begins when austenite is cooled to a temperature below MS on the time-temperature-transformation diagram. It is a diffusionless transformation achieved by the deformation of the parent lattice into that of the product.
These are both the products of the reconstructive transformation of austenite, but an allotriomorph forms at an austenite grain surface whereas an idiomorph nucleates somewhere within the grain, out of contact with the grain surface.
Since spheroidisation is driven by interfacial area, fine pearlite spheroidises more readily than coarse pearlite. Plastically deformed pearlite which is fragmented will also spheroidse relatively rapidly (Fig. 19).
The change in crystal structure can also be achieved in effect by breaking the bonds in the austenite and rearranging the atoms into the structure of ferrite whilst maintaining the overall shape. This requires atoms to diffuse over distances comparable to the size of the transformation product. Thus, although the strain energy associated with displacive transformations is avoided, this reconstructive mechanism can only occur at temperatures where atoms are sufficiently mobile. Given that atoms are mobile, certain species which are more soluble in a particular phase (α or γ) will tend to migrate preferentially into that phase, leading to a difference in the chemical composition between α and γ. The atomic correspondence between the parent and product phases is lost in a reconstructive transformation. The shape of the transformation product is either determined by growth circumstances, or as equilibrium is approached, by a minimisation of the overall interfacial energy per unit volume.
Figure 3 illustrates how the major transformation products can be classified according to the atomic mechanisms of transformation. The details of this and the relevance to the interpretation of microstructure will become apparent as this tutorial progresses. One example is that all the displacive transformation products necessairly have a plate shape.
Fig. 26 shows the form of the fracture surface expected when failure occurs due to impurity embrittlement at the prior austenite grain boundaries. The grains simply separate at the grain surfaces with little absorption of energy during fracture.
One characteristic of a reconstructive transformation is that the transformation product is not limited to the grain in which it nucleates. The ferrite (or pearlite) can grow across austenite grain boundaries. Thus, the allotriomorph shown in Fig. 7 thickens into both of the adjacent austenite grains. In contrast, displacive transformation products are confined to the grains in which they nucleate. This is because the discipline movement of atoms during transformation cannot be sustained across the austenite grain surfaces.
Fig. 23 shows an interference micrograph of a sample of austenite which was polished flat and then allowed to transform into martensite. The different colours indicate the displacements caused when martensite forms. This physical deformation is described on a macroscopic scale as an invariant-plane strain (Fig. 24) consisting of a shear strain s of about 0.25 and a dilatation δ normal to the habit plane of about 0.03.
Pearlite is a reconstructive transformation which always involves the diffusion of all elements including iron. It cannot happen in the absense of substantial atomic mobility. In alloy steels, in addition to interstitial carbon, the substitutional solutes will partition between the cementite and ferrite. Figure 17 shows this to be the case, with C, Mn and Cr enriching inside the cementite whereas Al and Si partition into the ferrite.
The Formula SAE Michigan competition was a chance for CUFSAE to showcase Tiger 24’s capabilities and their astounding efforts. 120 cars competed, making this one of the larger competitions CUFSAE has participated in. Of those 120 cars, the Tiger 24 displayed great competitive attributes finishing 20th in design and 60th overall.
In alloys containing large concentrations of solutes (for example, Fe-1C wt% or Fe-30Ni wt%), the plate shape of martensite is clearly revealed because substantial amounts of retained austenite are present in the microstructure, as illustrated in Fig. 27. In contrast, lower alloy steels transform almost completely to martensite when cooled sufficiently rapidly. Therefore, the microstructure appears different (Fig. 28) but still consists of plates or laths of martensite.
The strain energy per unit volume, E scales with the shear modulus of the austenite μ the strains and the thickness to length ratio c/r as illustrated in Fig. 24. The martensite therefore forms as a thin plate in order to minimise the strain energy. All of the displacive transformation products are therefore in the form of thin plates.
The phase diagram for obvious reasons does not feature time. The kinetics of transformation are better illustrated using a time-temperature-transformation (TTT) diagram as illustrated in Fig. 4. There are two "C" curves, the top one for reconstructive transformations and the lower one for displacive transformations. Also illustrated are schematic microstructures within individual austenite grains.
The Bain strain which converts austenite into martensite is a huge deformation; to mitigate its effects there are other deformations which accompany the transformation. These change the overall shape deformation into an invariant-plane strain. One consequence is that there are lattice invariant deformations such as slip and twinning on a fine scale. Slip simply leads to steps in the interface, whereas twinning also introduces interfaces inside the martensite plate, as illustrated in Fig. 32.
We have categorised transformations into displacive and reconstructive, with the former being strain dominated and the latter diffusion dominated. Displacive transformations are also known as military transformations by analogy to a queue of solidiers boarding a bus. The soliders board the bus in a disciplined manner such that there is a defined correspondence between their positions in the bus and those in the queue. Near neighbours remain so on boarding. There is thus no diffusional mixing and no composition change. Because the soldiers are forced to sit in particular positions, there will be a lot of strain energy and this is not an equilibrium scenario.
Imagine, as illustrated in Fig. 2, that the austenite consists of a mixture of square atoms and round atoms, and has the unit cell outlined in red. One way of changing the crystal structure is to do so without disrupting the relative order of the atoms. This can be done by generating the unit cell of ferrite by a homogeneous deformation of the parent γ. In this displacive mechanism, the overall shape of the sample must change in a manner consistent with the change in crystal structure. When this shape deformation occurs in the bulk of a polycrystalline steel, its accommodation leads to a lot of strain energy. This energy can be minimised if the ferrite adopts a thin-plate shape. Since transformation occurs by a deformation, the atoms maintain the sequence which existed in the parent phase. There is therefore, no change in the chemical composition during transformation. There is also a one-to-one atomic correspondence between the ferrite and austenite, which is the basis of the shape memory effect.
Isbainitestronger thanmartensite
Pearlite is in fact a mixture of two phases, ferrite and cementite (Fe3C. It forms by the cooperative growth of both of these phases at a single front with the parent austenite. In Fe-C systems, the average chemical composition of the pearlite is identical to that of the austenite; the latter can therefore completely transform into pearlite.
We shall interpret microstructrures in the context of the iron-carbon equilibrium phase diagram, even though steels inevitably contain other solutes, whether by design or as impurities. The diagram is nevertheless useful since the transformation behaviour of austenite does not change dramatically unless the steel has a large concentration of solutes.
We have emphasised that the discipline motion of atoms cannot be sustained across austenite grain boundaries and hence plates of martensite, unlike allotriomorphs, are confined to the grains in which they nucleate (Fig. 25).
The problem can be solved by using transmission electron microscopy to conduct the crystallographic analysis, however, this limits the size of the region than can be examined.
The interlamellar spacing within pearlite can be made fine by growing the pearlite at large thermodynamic driving forces. Figure 15 shows a transmission electron micrograph of pearlite where the interlamellar spacing is about 50 nm. This is well below the resolution of an optical microscope (typically 500 nm). It follows that the lamellae in this case cannot be resolved using optical microscopy, as illustrated in Fig. 16.
It is important to realise that a colony of pearlite is a bicrystal. Although a steel becomes stronger as the interlamellar spacing is reduced, it does not become tougher because the colony size is what represents the crystallographic grain size. Thus, a propagating cleavage crack can pass undeviated across a colony of pearlite.



 0086-813-8127573
0086-813-8127573