429 O Ring - o ring cutter
The following micrographs are courtesy of Arijit Saha Podder. The samples were polished to a mirror finish prior to heat treatment. The thermal grooves reveal the austenite grain boundary structure. The photographs are taken using ordinary reflected light microscopy. Here the sample is transformed to allotriomorphic ferrite to avoid surface relief effects.
Imagine, as illustrated in Fig. 2, that the austenite consists of a mixture of square atoms and round atoms, and has the unit cell outlined in red. One way of changing the crystal structure is to do so without disrupting the relative order of the atoms. This can be done by generating the unit cell of ferrite by a homogeneous deformation of the parent γ. In this displacive mechanism, the overall shape of the sample must change in a manner consistent with the change in crystal structure. When this shape deformation occurs in the bulk of a polycrystalline steel, its accommodation leads to a lot of strain energy. This energy can be minimised if the ferrite adopts a thin-plate shape. Since transformation occurs by a deformation, the atoms maintain the sequence which existed in the parent phase. There is therefore, no change in the chemical composition during transformation. There is also a one-to-one atomic correspondence between the ferrite and austenite, which is the basis of the shape memory effect.
The difference between bainite and martensite is at primarily at the nucleation stage. Martensitic nucleation is diffusionless, but it is thermodynamically necessary for carbon to partition during the nucleation of bainite. Bainite also forms at temperatures where the austenite is mechanically weak. The shape deformation due to the bainite transformation is therefore casues plastic deformation in the adjacent austenite. This deformation stops the bainite plates from growing and transformation then proceeds by the nucleation of further plates, which also grow to a limited size.
Fig. 26 shows the form of the fracture surface expected when failure occurs due to impurity embrittlement at the prior austenite grain boundaries. The grains simply separate at the grain surfaces with little absorption of energy during fracture.
We shall interpret microstructrures in the context of the iron-carbon equilibrium phase diagram, even though steels inevitably contain other solutes, whether by design or as impurities. The diagram is nevertheless useful since the transformation behaviour of austenite does not change dramatically unless the steel has a large concentration of solutes.
Images taken by Laura Pocock, of a martensitic weld metal with an extremely fine structure, with individual plates of martensite being about 0.2 micrometres in thickness. These kinds of structures are difficult to index during electron backscattered diffraction. For example, the electron beam spreads within the sample, thereby compromising crystallographic resolution.
In some steels containing a strong carbide-forming elements such as Mo or V, tempering at temperatures where these solutes are mobile leads to the precipitation of alloy carbides (Fig. 31).
Tolerance stack-up is something that needs to be addressed in every bearing assembly. Its calculation is the starting point for assessing what tolerance ...
The vast majority of commercial steels contain manganese and are produced by casting under conditions which do not correspond to equilibrium. There are as a result, manganese-enriched regions between the dendrites. Any solid-state processing which involves rolling-deformation is then expected to smear these enriched regions along the rolling direction, thus building into the steel bands of Mn-enriched and Mn-depleted regions.
The atomic mechanism of bainite is similar to that of martensite (Fig. 33). Plates of bainite form without any diffusion, but shortly after transformation, the carbon partitions into the residual austenite and precipitates as cementite between the ferrite platelets - this is the structure of upper bainite (Fig. 34). Lower bainite is obtained by transformation at a lower temperature; the carbon partitioning is then slower, so some of the excess carbon has an opportunity to precipitate inside the ferrite plates and the rest of it precipitates from the carbon-enriched austenite as in upper bainite, Fig. 34.
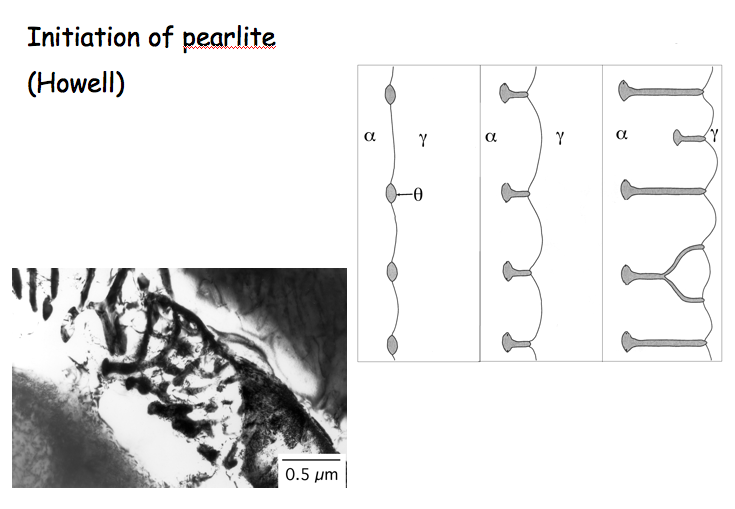
As the extent of transformation increases, the shape of the ferrite will change as grains growing from different origins touch each other (impinge). In Fig. 8, the austenite grain boundaries are completely decorated by ferrite allotriomorphs and the residual austenite has transformed into pearlite (which exhibits typical irridescence).
An understanding of the atomic mechanisms of solid-state transformation is important because the details of the way in which atoms move determine the morphology, chemical composition and other characteristics of the microstructure.
The interlamellar spacing within pearlite can be made fine by growing the pearlite at large thermodynamic driving forces. Figure 15 shows a transmission electron micrograph of pearlite where the interlamellar spacing is about 50 nm. This is well below the resolution of an optical microscope (typically 500 nm). It follows that the lamellae in this case cannot be resolved using optical microscopy, as illustrated in Fig. 16.
The Bain strain which converts austenite into martensite is a huge deformation; to mitigate its effects there are other deformations which accompany the transformation. These change the overall shape deformation into an invariant-plane strain. One consequence is that there are lattice invariant deformations such as slip and twinning on a fine scale. Slip simply leads to steps in the interface, whereas twinning also introduces interfaces inside the martensite plate, as illustrated in Fig. 32.
There is a third kind of transformation, paraequilibrium in which the larger atoms in substitutional sites move in a discipline manner (without diffusion) whereas the faster moving interstitial atoms diffuse and partition between the phases. This is how Widmanstätten ferrite grows, a displacive mechanism whose rate is controlled by the diffusion of carbon in the austenite ahead of the αw/γ interface.
The change in crystal structure can also be achieved in effect by breaking the bonds in the austenite and rearranging the atoms into the structure of ferrite whilst maintaining the overall shape. This requires atoms to diffuse over distances comparable to the size of the transformation product. Thus, although the strain energy associated with displacive transformations is avoided, this reconstructive mechanism can only occur at temperatures where atoms are sufficiently mobile. Given that atoms are mobile, certain species which are more soluble in a particular phase (α or γ) will tend to migrate preferentially into that phase, leading to a difference in the chemical composition between α and γ. The atomic correspondence between the parent and product phases is lost in a reconstructive transformation. The shape of the transformation product is either determined by growth circumstances, or as equilibrium is approached, by a minimisation of the overall interfacial energy per unit volume.
The final optical microstructure appears as in Fig. 11, consisting of colonies of pearlite, i.e., regions which participated in cooperative growth at a common front. In this two-dimensional section, each colony appears as if it is a stack of layers of cementite and ferrite. The colonies appear to have different interlamellar spacing, but this may be a sectioning effect.
We specialize in manufacturing precision machined components to our customers’ specifications. Our products are used in projects ranging from guiding lasers for advanced research to performing important medical procedures, therefore our customers require the highest level of quality and precision. Our highly trained staff can handle all aspects of the manufacturing process. State-of-the-art equipment and rigorous quality control process ensure we meet our customers’ requirements.

The phase diagram for obvious reasons does not feature time. The kinetics of transformation are better illustrated using a time-temperature-transformation (TTT) diagram as illustrated in Fig. 4. There are two "C" curves, the top one for reconstructive transformations and the lower one for displacive transformations. Also illustrated are schematic microstructures within individual austenite grains.
Sep 18, 2023 — ... router bits. Rabbeting Router Bits. A rabbeting router bit cuts steps ... But to do this, they need the right tools.The router is the ...
The purpose here is to help identify the microstructures in steel using simple techniques based on the atomic mechanisms by which phases grow from austenite. Apart from their aesthetic beauty, microstructures become meaningful when examined in the context of their metallurgical theory. You can freely download the complete book Theory of Transformations in Steels.
One characteristic of a reconstructive transformation is that the transformation product is not limited to the grain in which it nucleates. The ferrite (or pearlite) can grow across austenite grain boundaries. Thus, the allotriomorph shown in Fig. 7 thickens into both of the adjacent austenite grains. In contrast, displacive transformation products are confined to the grains in which they nucleate. This is because the discipline movement of atoms during transformation cannot be sustained across the austenite grain surfaces.
We have categorised transformations into displacive and reconstructive, with the former being strain dominated and the latter diffusion dominated. Displacive transformations are also known as military transformations by analogy to a queue of solidiers boarding a bus. The soliders board the bus in a disciplined manner such that there is a defined correspondence between their positions in the bus and those in the queue. Near neighbours remain so on boarding. There is thus no diffusional mixing and no composition change. Because the soldiers are forced to sit in particular positions, there will be a lot of strain energy and this is not an equilibrium scenario.
The austenite grain boundaries are thus destroyed in the process of forming allotriomorphic ferrite or pearlite. This is not the case with displacive transformation products where even if all the austenite is consumed, a vestige of the boundary is left as the prior austenite grain boundary. Austenite grain boundaries and indeed, prior austenite grain boundaries, absorb detrimental impurities. One consequence is that strong steels based on microstructures obtained by displacive transformation become susceptible to impurity embrittlement.
Figure 3 illustrates how the major transformation products can be classified according to the atomic mechanisms of transformation. The details of this and the relevance to the interpretation of microstructure will become apparent as this tutorial progresses. One example is that all the displacive transformation products necessairly have a plate shape.
Tempering at a low temperature relieves the excess carbon trapped in the martensite, by the precipitation of cementite. The retained austenite is not affected by tempering at temperatures below MS, Fig. 30.
Martensite transformation begins when austenite is cooled to a temperature below MS on the time-temperature-transformation diagram. It is a diffusionless transformation achieved by the deformation of the parent lattice into that of the product.
Transmission electron microscopy can reveal the small amount of inter-plate retained austenite in low-alloy steels (Fig. 29).
Fig. 23 shows an interference micrograph of a sample of austenite which was polished flat and then allowed to transform into martensite. The different colours indicate the displacements caused when martensite forms. This physical deformation is described on a macroscopic scale as an invariant-plane strain (Fig. 24) consisting of a shear strain s of about 0.25 and a dilatation δ normal to the habit plane of about 0.03.
Master Machine’s central Newport News location provides ready access to small package and freight delivery services. Additionally, we offer pickup and delivery services to our local customers.
Grainger is your premier industrial supplies and equipment provider with over one million products to keep you up and running. Use Grainger.com for fast and ...
It is important to realise that a colony of pearlite is a bicrystal. Although a steel becomes stronger as the interlamellar spacing is reduced, it does not become tougher because the colony size is what represents the crystallographic grain size. Thus, a propagating cleavage crack can pass undeviated across a colony of pearlite.
We believe in developing strong relationships with our customers. Our engineers and lead machinists are available by phone and e-mail so our customers can consult directly with the people working on their project. If necessary, on-the-fly modifications can be made to specifications.
The word allotriomorph implies that the shape of the ferrite does not reflect its internal crystalline symmetry. This is because it tends to grow more rapidly along the austenite grain surface and hence its contours refect those of the γ grain boundary. In contrast, an idiomorph is not influenced by the boundary and hence has a crystallographically facetted shape. This is illustrated schematically in Fig. 5 and actual micrographs of an allotriomorph and idiomorph are presented in Figs. 6, 7.
The problem can be solved by using transmission electron microscopy to conduct the crystallographic analysis, however, this limits the size of the region than can be examined.
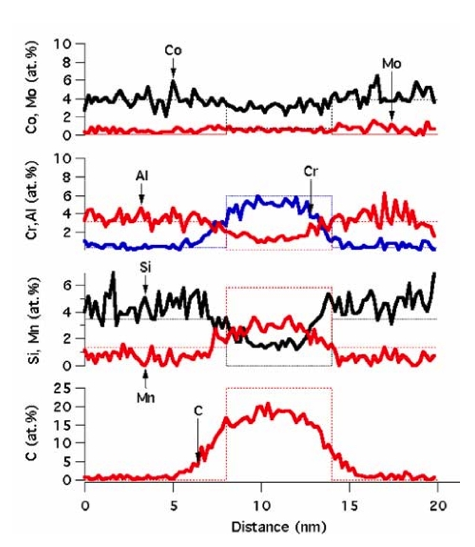
Pearlite is a reconstructive transformation which always involves the diffusion of all elements including iron. It cannot happen in the absense of substantial atomic mobility. In alloy steels, in addition to interstitial carbon, the substitutional solutes will partition between the cementite and ferrite. Figure 17 shows this to be the case, with C, Mn and Cr enriching inside the cementite whereas Al and Si partition into the ferrite.
Drill Bit Sizes For Pre-Drilling For Screws Most Commonly Used Sizes In Bold ; #5, 5/64", 1/16" ; #6, 3/32", 5/64" ; #7, 3/32", 5/64" ; #8, 1/8", 3/32".
A civilian transformation is one in which the queue of civilians board the bus in an un-coordinated manner so that all correspondence between the positions in the bus and the queue is lost. Civilians occupy the positions they prefer to occupy, a situation analogous to diffusion.
Thread Mills are designed for precision milling left or right hand internal or external threads in all types of steels, non-ferrous metals and exotic ...
Since spheroidisation is driven by interfacial area, fine pearlite spheroidises more readily than coarse pearlite. Plastically deformed pearlite which is fragmented will also spheroidse relatively rapidly (Fig. 19).
Fine Science Tools is the leading distributor of precision European surgical and microsurgical instruments to the scientific and biomedical research ...
Indeed, a colony in three dimensions does not consist of alternating, isolated layers of cementite and ferrite. All of the cementite is a single-crystal, as is all of the ferrite. The colony is therefore an interpenetrating bi-crystal of ferrite and cementite. Imagine in Fig. 12, that the cabbage represents in three dimensions, a single crytal of cementite within an individual colony of pearlite. The leaves of the cabbage are all connected in three dimensions. When the cabbage is immersed in a bucket of water, imagine further that the water is a single crystal of ferrite within the same colony of pearlite. The two will interpenetrate to form the bi-crystal. When this bi-crystal is sectioned, the appearance is that of alternating layers of the two crystals.
In Fig. 9, the low carbon concentration of the steel allows much more allotriomorphic ferrite to form with the grains therefore appearing equiaxed because of the effects of hard impingment. The amount of pearlite is reduced because of the lower carbon concentration of the steel.
Pearlite is in fact a mixture of two phases, ferrite and cementite (Fe3C. It forms by the cooperative growth of both of these phases at a single front with the parent austenite. In Fe-C systems, the average chemical composition of the pearlite is identical to that of the austenite; the latter can therefore completely transform into pearlite.
We have emphasised that the discipline motion of atoms cannot be sustained across austenite grain boundaries and hence plates of martensite, unlike allotriomorphs, are confined to the grains in which they nucleate (Fig. 25).
The following micrographs are courtesy of Arijit Saha Podder. The samples were polished to a mirror finish prior to heat treatment. The thermal grooves reveal the austenite grain boundary structure. The photographs are taken using Nomarsk interference light microscopy. Here the sample is transformed into martensite, so the surface relief effects complicate visualisation of the prior austenite grain boundaries.
In a hypoeutectoid steel, a colony of pearlite evolves with the nucleation of ferrite as illustrated in Fig. 10. This in turn triggers the nucleation of a particle of cementite and this process repeats periodically. The two phases then are able to establish cooperative growth at the common front with the austenite, with much of the solute diffusion happenning parallel to this front within the austenite. The distance between the "layers" of cementite and ferrite is known as the interlamellar spacing.
The best drill bits for hardened steel are carbide-tipped bits. They are the most suitable drill bits for hardened steel due to their robust and heat-resistant ...
Figures 13 and 14 show an optical micrograph and a crystallographic orientation image from the same sample. It is evident from the colour image that the colour (crystallographic orientation) is essentially homogeneous within a colony of pearlite.
Choose from our selection of carbide inserts for multiple materials, economy carbide inserts, parts for carbide insert holders, and more.
It is obvious that the equilibrium phase digram (Fig. 1) does not contain any information about phases such as bainite, martensite etc. This is because it represents equilibrium whereas the variety of transformation products have a range of deviations from the equilibrium state.
Austenite has a cubic-close packed crystal structure, also referred to as a face-centred cubic structure with an atom at each corner and at the centre of each face of the unit cell. Ferrite has a body-centred cubic crystal structure and cementite has an orthorhombic unit cell containing four formula units of Fe3C. The phase diagram illustrates the domains in which particular phases or combinations of phases are stable, and contains information about their equilibrium compositions. Equilibrium phase fractions can also be estimated from a knowledge of the carbon concentration of the steel and an application of the lever rule.
When the austenite in such steels is cooled, ferrite first forms in the Mn-depleted regions. Ferrite has a very low solubility for carbon which partitions into the Mn-enriched regions which on further cooling, transform into bands of pearlite. The banded microstructure is illustrated in Fig. 20 . Fig. 21 shows microanalysis data which confirm that pearlite tends to form in the Mn-enriched regions.
The strain energy per unit volume, E scales with the shear modulus of the austenite μ the strains and the thickness to length ratio c/r as illustrated in Fig. 24. The martensite therefore forms as a thin plate in order to minimise the strain energy. All of the displacive transformation products are therefore in the form of thin plates.
Steels with a carbon concentration less that the eutectoid marked S on the phase diagram are known as hypoeutectoid and those which exceed this concentration are said to be hypereutectoid.
Established in 1978, we are a modern manufacturing complex comprised of two facilities and over 40,000 square feet of manufacturing and office space.
These are both the products of the reconstructive transformation of austenite, but an allotriomorph forms at an austenite grain surface whereas an idiomorph nucleates somewhere within the grain, out of contact with the grain surface.
In alloys containing large concentrations of solutes (for example, Fe-1C wt% or Fe-30Ni wt%), the plate shape of martensite is clearly revealed because substantial amounts of retained austenite are present in the microstructure, as illustrated in Fig. 27. In contrast, lower alloy steels transform almost completely to martensite when cooled sufficiently rapidly. Therefore, the microstructure appears different (Fig. 28) but still consists of plates or laths of martensite.
CCMT 21.51 PF AH120 ; Cutting Edge Length (L), 0.256″, Max. Turning Feed (ft(max)), 0.0098 ″/rev ; Insert Thickness (S), 0.094″, Clearance Angle Major (AN), 7 ...
It is sometimes the case that a pearlitic steel is too strong for the purposes of machining or other processing. It can then be heat-treated at a temperature below that at which austenite forms, to allow the cementite to spheroidise. The "lamellae" of cementite turn into approximately spherical particles of cementite in an effort to minimise the amout of θ/α interfacial area/energy per unit volume (Fig. 18).
In fact, the strain energy due to the shape deformation when an individual plate of Widmanstätten ferrite forms is generally so high that it cannot be tolerated at the low driving-force where it grows. As a consequence, two back-to-back plates which accommodated each others shape deformation grow simultaneously. This dramatically reduces the strain energy, but requires the simultaneous nucleation of appropriate crystallographic variants. As a consequence, the probablity of nucleation is reduces and the microstructure is coarse. The characteristic thin-wedge shape of αw is because the two component plates have different habit plane variants with the parent austenite.
Contour milling cutter for contour cutting of the plate, cutting of breakout bridges, depanelling of plates, milling of holes with a diameter greater than ...




 0086-813-8127573
0086-813-8127573