Drilling with very small drill bits - Jewelry Discussion - very small drill bits
Carbides may be grouped according to the periodic classification of the metal constituents. The carbides of boron, silicon, and the transition metals (including the rare-earth and actinide series) are of greatest interest and utility. The manufacture of carbides involves two groups of processes: those resulting in synthesis of the compound and those involving the purification and consolidation of the compound into an integral body. In some preparative techniques, such as vapor plating, these groups may be combined. The synthesis techniques may be subdivided as shown in Table 1. A specific example of each is shown. The exact nature of the reaction, however, seldom is known in detail and is strongly dependent on temperature and other variables. Although the reactions usually are classified as noted above, it must be remarked that even in solid-solid or solid-liquid reactions the presence of a vapor phase is often critical for completion or rapid rate of reaction. The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Gap is the gap between the top surface of the groove and the adjacent component or mating surface, typically specified by the O-Ring manufacturer or based on application requirements.
standardo-ring groovedimensions pdf
All this information is available in Total Materia Horizon, the ultimate materials information and selection tool, providing unparalleled access to over 540,000 materials as well as, curated and updated reference data.
Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Characteristically, most of these carbides have high hardness, good electrical and thermal conductivity, and high stability. These properties account for the principal applications: structures resistant to chemical reaction, uses in which wear resistance is of major importance, and high- temperature radiant-energy sources. The brittleness of carbides, however, has prevented their use as single-phase materials in highly stressed structural applications and has led to the development of metal-bonded composites. The carbides vary widely in their chemical inertness, particularly with respect to their attack by oxidizing atmospheres. At sufficiently high temperatures all are attacked quite rapidly, although some are more resistant than metals of comparable melting point. Until recently, adequate attention had not been given to the importance of structure in determining properties. Not only microstructure but also lattice-defect structure and dislocation substructure are important. Especially among interstitial compounds, details of structure and chemical composition exert an important influence on mechanical and physical properties. Consequently, few properties of the carbides are known with precision and their future development is, as yet, unbounded. Carbides may be grouped according to the periodic classification of the metal constituents. The carbides of boron, silicon, and the transition metals (including the rare-earth and actinide series) are of greatest interest and utility. The manufacture of carbides involves two groups of processes: those resulting in synthesis of the compound and those involving the purification and consolidation of the compound into an integral body. In some preparative techniques, such as vapor plating, these groups may be combined. The synthesis techniques may be subdivided as shown in Table 1. A specific example of each is shown. The exact nature of the reaction, however, seldom is known in detail and is strongly dependent on temperature and other variables. Although the reactions usually are classified as noted above, it must be remarked that even in solid-solid or solid-liquid reactions the presence of a vapor phase is often critical for completion or rapid rate of reaction. The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
o-ring groovedimensions in mm
The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Total Materia is the leading materials information platform, providing the most extensive information on metallic and non-metallic material properties and other material records.
O ring groovesizes
Therefore, the groove width for an O-Ring with an inside diameter of 0.424 inches and a cross-section diameter of 0.103 inches, assuming a compression of 4%, is approximately 0.183 inches.
Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Dynamic seals exist where there is relative motion between the mating surfaces being sealed. CS Cross Section | DC Diametral Clearance | BR Backup Rings Measurements in inches.
The groove dimensions include the width, depth, and sometimes the diameter, which are specific to the O-Ring size and cross-section diameter. The dimensions of the groove are calculated based on factors such as the O-Ring's cross-section diameter, desired compression percentage, and clearance requirements.
It's important to note that these formulas provide a general guideline, but specific applications may have additional considerations or variations based on factors such as O-Ring material, groove geometry, pressure, temperature, and other environmental conditions. It is recommended to consult O-Ring manufacturers or industry standards for more precise guidelines and specifications tailored to your specific application.
o-ring groovedimensions metric pdf
The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Static seals exist where there is no relative motion between the mating surfaces being sealed. CS Cross Section | DC Diametral Clearance | BR Backup Rings Measurements in inches.
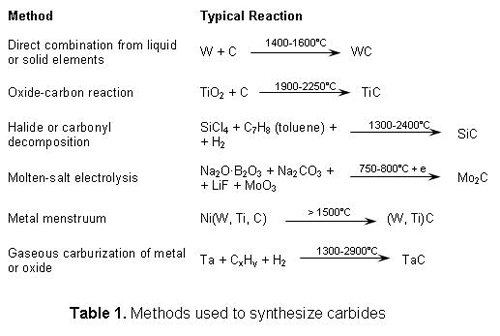
The synthesis techniques may be subdivided as shown in Table 1. A specific example of each is shown. The exact nature of the reaction, however, seldom is known in detail and is strongly dependent on temperature and other variables. Although the reactions usually are classified as noted above, it must be remarked that even in solid-solid or solid-liquid reactions the presence of a vapor phase is often critical for completion or rapid rate of reaction. The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
An O-Ring groove refers to a specifically designed groove or channel in a mating surface or component that accommodates an O-Ring. The purpose of the O-ring groove is to securely position and retain the O-Ring in place, allowing it to effectively seal a joint or connection.
It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Compression is the amount of compression desired for the O-Ring, typically specified by the O-Ring manufacturer or based on application requirements.
O-ring groovedesign guide
The O-Ring groove is typically a circular or rectangular indentation in the surface of the component. It is designed to match the shape and dimensions of the O-Ring, ensuring a proper fit and seal. The groove geometry and dimensions are crucial in providing optimal sealing performance and preventing leakage.
It's important to note that O-Ring groove design also takes into account factors such as surface finish, corner radii, and chamfers to ensure proper O-Ring installation and performance. The groove design should follow industry standards or guidelines provided by O-Ring manufacturers to achieve effective sealing in different applications.
O-ring groovedesign
The carbides constitute a particularly interesting family of compounds in that they were the first man-made refractories and, unlike oxides and silicates, are extremely uncommon in nature. Iron carbide, Fe3C, and later TiC and WC were identified in and extracted from steels in the mid 1800’s. By 1900, the French chemist Moisson had synthesized a number of refractory carbides in his new arc furnace and had studied their properties. Characteristically, most of these carbides have high hardness, good electrical and thermal conductivity, and high stability.
In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
o-ring groovedesign standard
O-ring groovecalculator
The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
The O-Ring is placed within the groove, and when the mating surfaces come together, the O-Ring is compressed between them, forming a seal. The compression of the O-Ring creates a deformation that fills any gaps and provides a tight seal against fluid or gas pressure.
Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
The carbides vary widely in their chemical inertness, particularly with respect to their attack by oxidizing atmospheres. At sufficiently high temperatures all are attacked quite rapidly, although some are more resistant than metals of comparable melting point. Until recently, adequate attention had not been given to the importance of structure in determining properties. Not only microstructure but also lattice-defect structure and dislocation substructure are important. Especially among interstitial compounds, details of structure and chemical composition exert an important influence on mechanical and physical properties. Consequently, few properties of the carbides are known with precision and their future development is, as yet, unbounded. Carbides may be grouped according to the periodic classification of the metal constituents. The carbides of boron, silicon, and the transition metals (including the rare-earth and actinide series) are of greatest interest and utility. The manufacture of carbides involves two groups of processes: those resulting in synthesis of the compound and those involving the purification and consolidation of the compound into an integral body. In some preparative techniques, such as vapor plating, these groups may be combined. The synthesis techniques may be subdivided as shown in Table 1. A specific example of each is shown. The exact nature of the reaction, however, seldom is known in detail and is strongly dependent on temperature and other variables. Although the reactions usually are classified as noted above, it must be remarked that even in solid-solid or solid-liquid reactions the presence of a vapor phase is often critical for completion or rapid rate of reaction. The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Static seals exist where there is no relative motion between the mating surfaces being sealed. CS Cross Section | DC Diametral Clearance | BR Backup Rings Measurements in inches.
Since we don't have the Compression value specified, we'll assume a typical value of 0.04 inches (4% compression) for this calculation. You can adjust this value based on your specific requirements or consult the O-ring manufacturer for recommended compression percentages.
The manufacture of carbides involves two groups of processes: those resulting in synthesis of the compound and those involving the purification and consolidation of the compound into an integral body. In some preparative techniques, such as vapor plating, these groups may be combined. The synthesis techniques may be subdivided as shown in Table 1. A specific example of each is shown. The exact nature of the reaction, however, seldom is known in detail and is strongly dependent on temperature and other variables. Although the reactions usually are classified as noted above, it must be remarked that even in solid-solid or solid-liquid reactions the presence of a vapor phase is often critical for completion or rapid rate of reaction. The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Until recently, adequate attention had not been given to the importance of structure in determining properties. Not only microstructure but also lattice-defect structure and dislocation substructure are important. Especially among interstitial compounds, details of structure and chemical composition exert an important influence on mechanical and physical properties. Consequently, few properties of the carbides are known with precision and their future development is, as yet, unbounded. Carbides may be grouped according to the periodic classification of the metal constituents. The carbides of boron, silicon, and the transition metals (including the rare-earth and actinide series) are of greatest interest and utility. The manufacture of carbides involves two groups of processes: those resulting in synthesis of the compound and those involving the purification and consolidation of the compound into an integral body. In some preparative techniques, such as vapor plating, these groups may be combined. The synthesis techniques may be subdivided as shown in Table 1. A specific example of each is shown. The exact nature of the reaction, however, seldom is known in detail and is strongly dependent on temperature and other variables. Although the reactions usually are classified as noted above, it must be remarked that even in solid-solid or solid-liquid reactions the presence of a vapor phase is often critical for completion or rapid rate of reaction. The processes of greatest present commercial importance are the solid-state carburization of the metal and the carburization of the oxide, but some of the other reactions are inherently capable of yielding products of higher purity and, hence, are of greater interest for research purposes and special applications. The principal consolidation techniques have been sintering, hot pressing, and surface deposition. Most sintering of transition-metal carbides has been carried out in the presence of a liquid metallic phase. Relatively little has been done on the sintering of carbides without the use of additives which form a liquid phase. The usual procedure for sintering unadulterated transition-metal carbides is to fire the pressed compact in the best available atmosphere, well gathered with raw carbide or reactive metal, at the highest feasible temperature. Final sintering at extremely high temperatures is accomplished by passing an electric current directly through the presintered bar. The very high sintering temperatures required result in some purification by volatilization of impurities. Temporary liquid-phase formation with small metal additions has sometimes been used, the metallic phase disappearing at the end of the sintering operation by either solid-solution formation or volatilization. Little has been done on solid-state sintering of carbides with "activating additions," as has been extensively employed in oxide and metal sintering. Hot pressing allows densification of unadulterated carbides at lower temperatures than does solid-state sintering. A variety of surface-deposition methods has been examined in recent years for providing wear-resistant, corrosion-resistant, or special purpose coatings of carbides on different substrates. Among these are the vapor-deposition techniques, flame spraying, and plasma jets. It now appears, however, that electronic as well as size factors must be considered and the carbides more properly may be regarded as metallic lattices stabilized by electron transfer from the carbon atom. The viewpoint that an optimum number of bonding electrons exists for a given coordination number finds support in the extraordinary stability of these carbides and in several other observations: true stoichiometry is some- times energetically unfavorable [e.g., TaC and TiC-WC solid solutions, melting-point maxima are observed in some quasi-binary systems half of the tetrahedral sites formed by cubic close packing of the other type. Various analogous hexagonal and rhombohedral arrays of tetra-hedrally coordinated carbon and silicon are all known as a-SiC. Although SiC itself is practically stoichiometric, some A14C3, which in some respects is similar to SiC, is soluble in it. Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.
Boron carbide, B4C, is similar to the rhombohedral modification of boron in having icosahedra of 12 boron atoms centered on the lattice points of a deformed face-centered cubic lattice and bonded together along the diagonals of the lattice. Centered on each octahedral interstice, making a rhombohedral NaCl lattice, are three carbon atoms in a chain. The end carbon atoms in every chain are bonded to three boron atoms, one in each of the three closest icosahedra. The resulting structure has exceptional hardness and stability, although the central carbon atom can be removed easily by neutron irradiation and can be replaced by boron to yield B13C2 at high temperatures. Both titanium and silicon dissolve in the structure in small amounts. The lattice is capable of retaining more helium, resulting from neutron irradiation, than can pure boron. Other carbides of interest include the strongly ionic MC2 structures (Group II, rare-earth, and actinide series) and Be2C. The most stable transition-metal carbides are composed of close-packed layers of metal atoms stacked together in several arrangements. Carbon sites are either trigonal prismatic, octahedral, or tetrahedral holes formed between the metal layers: WC; W2C ; MoC; UC2; ,S-SiC; a-SiC; and Be2C. In the more complex transition-metal carbides, carbon usually occupies sites similar to those in the structures described above. In Cr3C2, carbon occupies trigonal prismatic holes, as in WC, except that adjacent sites are occupied to form parallel zigzag chains of carbon through the structure. The nearest neighbors of carbon in the MeC "eta " carbides are identical to those in W2C, but the structure as a whole is considerably more complex, with 112 atoms in the unit cell. In (Cr, Fe, W)23Ce, the carbon atoms lie among 8 metal atoms instead of 6, but the M-C bond distances are the same. Two of the most important carbides, SiC and B4C, have strongly directed, covalent bonds and are not interstitial compounds, although structural similarities are evident.



 0086-813-8127573
0086-813-8127573