HELICAL TOOLS FOR LOCKS - helical tools
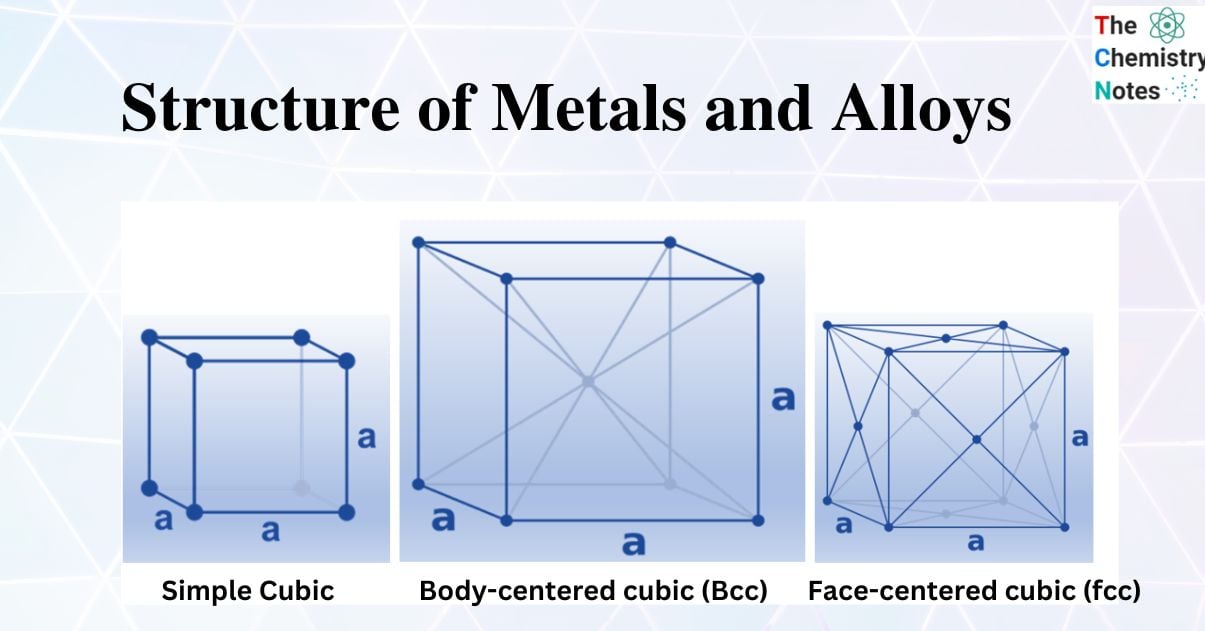
The name of this structure is simple cubic packing. In this construction, every sphere touches four other spheres that are identical to it. Additionally, it makes contact with spheres in the planes above and below, respectively. In this arrangement, each atom can create bonds with its six closest neighbors. Thus, it is claimed that each sphere has a coordination number of 6. It is inefficient to use space in a straightforward cubic form. In a straightforward cubic construction, the spheres actually occupy just 52% of the available area. The remaining area is empty. Only one element crystallizes in an easy cubic shape, polonium, due to the inefficiency of this structure.
(8 corner atoms × ⅛)+ (6 face atoms × ½)= 4 atoms/cell. This structure has the highest efficient packing (74%), along with its hexagonal relative (hcp). Many metals have either a fcc or a hcp structure.
(8 corner atoms × ⅛) + (1 center atom × 1)= 2 atoms/cell. The structure is common for alkali metals and early transition metals, and the packing is more efficient (68%). These structures are also used by alloys such as brass (CuZn).
In the cubic closest-packed structure, the atoms in these planes are oriented in different directions. Both the hexagonal and the cubic structures are equally effective when packed closely together. (Both take up 74% of the space.) Many metals crystallize in a cubic closest-packed form, including Ag, Al, Au, Ca, Co, Cu, Ni, Pb, and Pt. When these gases are cooled to low enough temperatures to solidify, they all do as well, with the exception of helium.
Since metal atoms are roughly shaped like spheres, they do not pack completely tightly and has some gaps in between the spheres. The packing efficiency of different unit cells varies. The number of atoms in the unit cell only includes the percentages of atoms contained within the box. Atoms in the unit cell’s corners count as 18 of an atom, atoms on the face count as 12, and an atom in the center counts as a complete atom. Let’s use this to figure out how many atoms are in a simple cubic unit cell, a face centered cubic (fcc) unit cell, and a body centered cubic (bcc) unit cell.
The simplest repeating unit in a cubic closest-packed structure is the face-centered cubic unit cell. In fact, the structure’s name, cubic closest-packed, derives from the fact that it contains face-centered cubic unit cells.
Some titles reflect further development of machinist skills such as tool and die maker, patternmaker, mold maker, programmer, and operator. A machinist is one who is called on to fix a problem with a part or to create a new one using metals, plastics, or rarely, wood. Depending on the company, a machinist can be any or all of the titles listed above.
The atoms in a metal lattice form a pattern that may be represented as a 3D box shape known as the unit cell, which repeats over the entire metal.
This occurs because the molten metal often contains a large number of crystallization nuclei that are dispersed. When four atoms are able to form a unit cell and lose enough thermal energy, such nuclei may arise. As more metal atoms get sufficiently low in energy to join in, these unit cells will expand, leading to the development of crystals. Homogeneous nucleation is the name given to this procedure.
It is known as a hexagonal closest-packed structure because it is made up of spheres that are alternately packed in hexagons on two planes. Each sphere makes contact with six spheres in the same plane, three spheres in the plane below, and three spheres above. In a hexagonal closest-packed arrangement, 74% of the available space is occupied, therefore making the coordination number 12. The hexagonal closest-packed structure is crucial for low-temperature materials like Be, Co, Mg, and Zn as well as the rare gas He because there is no known more effective way to pack spheres. Hence, the packing density of the hcp is 74%, just like the fcc structure.
Alloys include metals including brass, pewter, phosphor bronze, amalgam, and steel. The microstructure of complete solid solution alloys is a single solid phase. Depending on the thermal history, partial solutions produce two or more phases that may or may not be homogenous in distribution. The properties of an alloy are frequently distinct from those of its constituent elements.
8 corner atoms × ⅛ = 1 atom/cell. The packing in this structure is not efficient (52%) and so this structural type is extremely rare in metals.

Any group of substances distinguished by strong electrical and thermal conductivity, as well as malleability, ductility, and high light reflectance. A metal is a material with a lustrous look that conducts electricity and heat reasonably well when freshly manufactured, polished, or shattered. Metals are ductile (they may be formed into wires) and malleable (they can be hammered into thin sheets). These qualities are caused by the metallic link formed between the metal’s atoms or molecules.
More frequently, the presence of contaminants in the melt causes solidification to begin. Crystals start to form as the temperature falls below the melting point and as a result metal atoms start to accumulate on these impurities. Heterogeneous nucleation is the name given to this phenomenon. When all of the metal has formed, the crystals (also known as grains) will stop growing. They will start to collide as they expand, thus creating boundaries between the crystals where the atoms are placed erratically. This border, known as the grain boundary, is basically a defect in the metal’s crystal structure.
While the foregoing were primarily the materials that a machinist would be cutting, the cutters that the machinist uses must be harder and tougher than the materials to be cut. The materials in the cutters a machinist uses are most commonly high-speed steel, tungsten carbide, ceramics, Borazon, and diamond.[3]
In Australia, a related profession is a fitter and turner. A fitter and turner is the tradesperson who fits, assembles, grinds and shapes metal parts and subassemblies to fabricate production machines and other equipment.[1]
Crystals are three-dimensional repeating patterns. The fundamental repeating unit of the crystal is called the unit cell. It is a three-dimensional shape that may be replicated indefinitely using unit translations to fill holes in the structure.
Under the machinist title are other specialty titles that refer to specific skills that may be more highly developed to meet the needs of a particular job position, such as fitter (assembles parts), turning hand, mill hand, and grinder.
Ligands are the atoms, ions, or molecules that are linked to the main atom (or molecule/ion). When determining the coordination number of a central atom in a crystal, the calculation of the ligancy of molecules differs.
Individual crystal clusters, or “grains,” make up the interior structure of a metal. These grains’ shape, size, and orientation are a function of the alloy’s composition and manufacturing process (e.g. forging, casting or additive manufacturing). When the molten material solidifies, grains are created. These grains interact with one another as well as with phases and contaminants. The grain structure is typically adjusted for the technological use. Chiefly, the mechanical and technical qualities of these materials are closely related to the size, orientation, and other structural features of the grains. Additionally, future outside stimuli affect structural properties. These factors comprise:
A machinist is usually called upon when a part needs to be produced from a stock material by cutting. Such a part may be unique or may be needed in the thousands. The part could be anything made from metal or plastic, though machined parts are usually ones that require high precision and cannot be produced by other means. Machinists generally start with a saw cut length of stock or a casting. Producing a part will often require several steps and more than one machine tool. Each machine tool plays a specific role in cutting away excess material. When large numbers of parts are needed, production planning is required to plan the most logical workflow through a series of machines. Computer numerical controlled (CNC) machines are computer-driven tools that can machine a large variety of shapes, and whose use in the workflow depends on the part to be machined.[2]
Steel (iron + carbon) is a typical alloy used in building materials. This makes sense because it can hold more weight, is less likely to corrode, and can be molded more easily than iron.
The machine trade is an extremely broad field with a wide variety of workplaces, job duties, and types of work. Most machinists work in machine shops and factories where they operate machinery that produce precision component parts. In general, the occupation is exacting, and requires extensive knowledge of the tools and processes in order to achieve the tight tolerances and surface finishes that these parts specify.
You may determine a material’s characteristics, such as its strength, hardness, and ductility, by looking at the grain structure, also known as the microstructure.
The hexagonal or rhombic unit cells of this pattern would be replaced in three dimensions by three-dimensional boxes that would stack together to occupy all space.
Alloys are distinct. The type of alloy determines the atomic structure: substitutional or interstitial.In a substitutional alloy, the atoms of one metal are substituted with those of another. These new atoms are similar in size to the atoms of the other metals.The atoms of the second metal in an interstitial alloy are substantially smaller than those of the pure, original metal. These smaller atoms fit into the original structure’s “holes.”
Additive machining means 3D printing to create industrial components, prototypes, tooling, and end-use production parts. Additive machining comes into its own in the manufacturing of very small intricate parts, which could not be produced through any other manufacturing process. There are several processes in additive manufacturing which include direct metal deposition: electron beam melting, fused filament fabrication, select laser sintering, and variations of them.
Usually, alloys are created to “maximize” particular properties. The following are the distinctions between metals and alloys: Harder than component metals.
Because each sphere touches four spheres in the plane above and four more in the plane below, positioned toward the corners of a cube, this arrangement is known as a body-centered cubic structure. The body-centered cube is the repeating unit in this construction and is made up of an eight-sphere cube with a ninth identical sphere in the middle. This structure’s coordination number is 8. Body-centered cubic packing utilizes space more effectively than simple cubic packing; in this construction, 68% of the available space is used. A number of the early transition metals, including Ti, V, Cr, Mo, W, and Fe, pack in a body-centered cubic form, as do all of the metals in Group IA (Li, Na, K, and so forth), the heavier metals in Group IIA (Ca, Sr, and Ba), and the metals in Group IIA.
When the liquid material solidifies, the grains themselves are produced. Generally, for the use of the metal alloy, the grain structure is modified. Cupro-nickel, for instance, has a grain structure that allows the metal to be crushed into nickels and dimes.
Metals are made up of atom aggregates that are consistently organized in a crystalline structure. Metals and alloys are typically crystalline in structure. Generally, pure metals have fairly basic crystal structures with cubic or hexagonal unit cells, as we will see. Alloy crystal formations, on the other hand, can be quite complex.
Metals account for around three-quarters of all known chemical elements. Aluminum, iron, calcium, sodium, potassium, and magnesium are the most prevalent in the Earth’s crust. Although the great majority of metals are found in ores (mineral-bearing substances), a few, such as copper, gold, platinum, and silver, are usually found in their free state because they do not readily react with other elements.
It is simple to see why metals form structures that are tightly packed in hexagonal or cubic shapes. These structures have the highest possible coordination numbers, which enables each metal atom to establish bonds to the greatest number of nearby metal atoms, in addition to making the most use of available space.
Many machinists make mass-produced parts using highly automated computer numerical control machines which are common today, but still require such professionals to set up and calibrate the machines. Other more specialised machinists produce custom-made parts for prototyping, repair, or research. A machinist may work on manufacturing something relatively simple like a bracket, or a shaft, or something extraordinarily complex, such as aerospace components accurate to 5 micrometres.
CNC machines are becoming the standard due to their speed, precision, flexibility, repeatability, and reduced downtime while changing jobs. Production runs consisting of large numbers of parts are more cost effective and commonly referred to as production work in the trade. Conversely, small production runs are sometimes referred to as prototype or jobbing work.
Good machinists are highly sought after and respected skilled trades persons and are generally well-paid. In utility, medical, and military use companies, experienced machinists can earn over $100 000 per year.
A machinist is a tradesperson or trained professional who operates machine tools, and has the ability to set up tools such as milling machines, grinders, lathes, and drilling machines.[clarification needed]
Production engineers use blueprints and engineering drawings to produce detailed specifications of the part, especially its geometry (shape), then decide on a strategy to make it. Machine tools are then configured by the machinist and production commences. The machinist works with the quality department to ensure the specifications are maintained in the finished product.[2]
A competent machinist should have a well-developed mechanical aptitude, the ability to correctly use precision measuring instruments and to interpret blueprints, and a working knowledge of the proper parameters required for successfully utilizing the various tools commonly used in machining operations. CNC (computer numerical control) is the modern manufacturing method in which machinist use a form of programming called G-code to make components for a wide variety of industries. CNC programming is a highly skilled position. Programmers are usually machinist as well. A CNC programmer creates programs using software called CAM (computer aided manufacturing). The programmer must be proficient in math, speeds and feeds, machine tooling, work holding, and the different ways various materials react to stress and heat in the machining process.
The most common materials that machinists make parts from are steel, aluminium, brass, copper, and various alloys of these materials. Other less common materials such as vanadium, zinc, lead, or manganese are often used as alloying elements for the most common materials. Materials that machinists work with occasionally are plastics, rubber, glass, and wood products. Rarely, machinists also work with exotic and refractory metals. The term exotic metals is a general term describing out of the ordinary, rare or special purpose metals. A synonym might be space-age. A list of exotic metals might include, but is not limited to, titanium, beryllium, vanadium, chromium, molybdenum and tungsten, as well as special high-temperature metal alloys like Inconel or Hastelloy (superalloys). Very often the meaning of the term suggests the need for specialized handling and/or tooling to machine them effectively.[3]
Aggregates of atoms that are regularly organized in a crystalline structure make up metals. In contrast to what we have discussed thus far, which is the production of single crystals, metals typically form from a myriad of tiny crystals rather than from a single solidification from the melt.
The total number of atoms, ions, or molecules linked to a certain atom is indicated by the atom’s coordination number in a given molecule or crystal. An other name for an atom’s coordination number is “ligancy.”
Machinists usually work to very small tolerances, usually within 0.010" or 0.25 mm (more commonly expressed as ±0.005" (Plus or minus five thousandths of an inch) or ±0.13 mm), and sometimes at tolerances as low as +/-0.0001" (plus or minus one tenth of a thousandth of an inch – or 0.0025 mm) for specialty operations. A machinist deals with all facets of shaping, cutting and some aspects of forming metal, although forming is typically a separate trade. The operations most commonly performed by machinists are milling, drilling, turning, and grinding. There are other more specialized operations that a machinist will less frequently be called upon to perform such as honing, keyseating, lapping, and polishing, to name a few.
Alloys include metals including brass, pewter, phosphor bronze, amalgam, and steel. The microstructure of complete solid solution alloys is a single solid phase. Depending on the thermal history, partial solutions produce two or more phases that may or may not be homogenous in distribution. In general, the properties of an alloy are frequently distinct from those of its constituent elements.
Large commercial organizations often staff machinists on site in a maintenance mode to ensure continuing operations of the production machinery. Such machinists can often make replacement parts the same day. Because of this, the labor cost for this role are significantly lower than costs involved with production shutdowns.




 0086-813-8127573
0086-813-8127573