Mitre Lock Cutters - Cutters - Products - 1/4 round cutting tool
Chemical properties of nickelperiodic table
Inconel Alloy : Nickel makes up the biggest portion of the elements that are utilized in Inconel alloys, with chromium coming in second. These alloys can withstand extreme stresses, are resistant to corrosion and oxidation, and can be used in harsh situations. It is used for the fabrication of combustion chambers, thermal reactors, insulating cans, refractory cans, gas turbines, chemical production, and many more.
Cupro Nickel : It has excellent thermal conductivity, is incredibly ductile, has exceptional tensile strength, and is particularly resistant to corrosion caused by seawater. It is used for military equipment ,piping and heat exchangers ,desalination plants ,propellers and propeller shafts,etc.
Where isnickelfound
Uses Of Nickel in Aerospace, Marine and Military : The First and second world wars, as well as the subsequent cold wars, brought nickel to attention. Weapon manufacturing increased throughout this time, and different countries engaged in fierce warfare to establish their control.The use of nickel-based alloys is on the rise in the aircraft sector as well. Warships, aircraft armor plates, and tank armor all require a particular use of nickel. NASA has used it to protect spacecraft from the sun’s heat. It has a great resistance to corrosion, making it perfect for use in the construction of marine vehicles.
Chemical properties of nickelpdf
Who discoverednickel
Nickel is a hard, silver-white metal that can be found in soil, water, and foods like almonds, dried beans, and chocolate. Nickel is used by humans for a wide variety of purposes. A very small amount of nickel is required by the body. In several bodily chemical reactions, nickel is a necessary nutrient.Its particular bodily roles are not well understood.
Nickel is most commonly used in cars, the stainless steel found in cars is nickel alloy. due to the high corrosion resistance auto industry depends on stainless steel. It also has the ability to absorb energy from crashes. It is also lighter and more sturdy when compared to other metals.
Uses Of Nickel in Electronics : Electronic equipment and electric cars that employ nickel batteries frequently contain nickel. This metal is also used in large electronic components and the development of nanotechnology. Parts of laptops and smartphones contain nickel. Nickel-coated layers can also be found on compact discs.
Fluorine gas, F2, and nickel metal do interact, however, the reaction is sluggish. As a result, nickel is a valuable metal for fluorine storage tanks.Nickel metal reacts with chlorine (Cl2), bromine(Br2), or iodine(I2) to produce dichloride, NiCl2, dibromide, NiBr2, and diiodide, NiI2.
Physicalproperties of nickel
Invar Alloy : This alloy of iron and nickel has a small coefficient of thermal expansion as its main characteristic. It is mostly utilized in clocks, seismic creep gauges, valves in engines, large aerostructure molds, etc.
Top 10 usesof nickel
Usesof nickel
Information on nickel’s effects on species other than humans is not very abundant. We are aware that elevated nickel concentrations in sandy soil can obviously hurt plants while elevated nickel concentrations in surface waters can inhibit the growth of algae.Microorganisms can also experience growth reduction when nickel is present, but they often become resistant to nickel over time.
When nickel metal is slowly dissolved in diluted sulfuric acid, solutions containing the ionized Ni(II) and hydrogen gas, H2, are created. In practice, the complex ion [Ni(OH2)6]2+ represents the Ni(II).
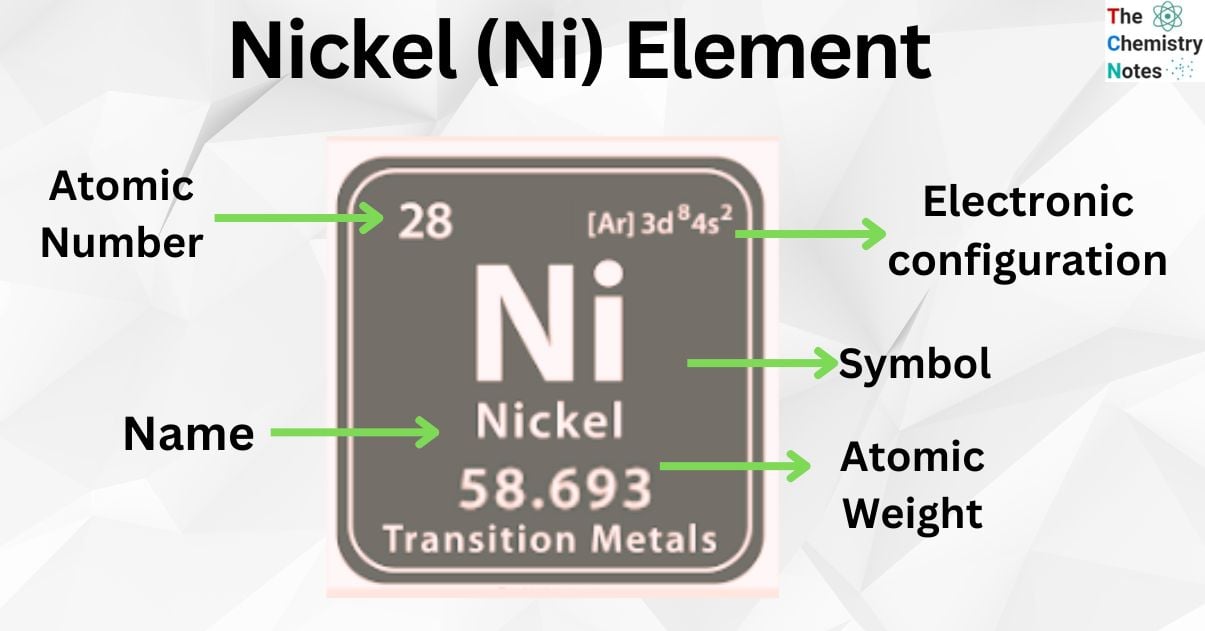
Nickel is the metallic element with the atomic number 28 and is represented by the symbol ‘Ni’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 10 (X) of the periodic table. It is a silvery-white lustrous metal with a slight golden tinge which has important magnetic properties.
What is nickel? What is so special about this essential metal? Where does it come from? And what is it used for? With this video, we take you on a quick tour of the world of nickel.
One of the most sought-after and used metals in contemporary industry is Nickel. Nickel (Ni) is a transition metal that is used in numerous applications. What distinguishes it from other metals is its adaptability. There are many other uses for it, but only a handful are covered below.
Nickel is being used as a superalloy, a type of high-temperature metal substance that can function under specific stress conditions. Corrosion-resistant high-temperature alloys with nickel bases are frequently employed at service temperatures over 500°C. Since their introduction in the 1950s, nickel-base superalloys have been widely employed for high-temperature applications in aerospace, power generation, and automotive.
Nickel is thought to be the second most common element in the earth’s core, right behind iron. It is 22nd most abundant element in Earth’s crust. Overall it is the 5th most abundant element on Earth. Magnetic sulfide deposits and laterite deposits, which are the by products of severe weathering of nickel-rich rocks on the surface, are the two main types of deposits where nickel typically occurs.
3chemical properties of nickel
The reaction of metal with oxygen (O2) seems to slow down at higher temperatures and create some nickel (II) oxide instead.
Under normal conditions, nickel doesn’t react with air. However, when it is finely split metal reacts well with air and can become pyrophoric during the period.
Nichrome Alloy : This nickel-chromium alloy has excellent electrical resistance as well as elevated temperature resistance as its main characteristics. Its color is silvery-gray. Its primary applications include resistance wire, heating elements for appliances like space heaters and toasters, and dental restorations.
Nickel is used in electroplating. It increases wear resistance and inhibits corrosion. The overall thickness of the plate is additionally increased. Nickel improves the appearance and brightness of an object from an aesthetic standpoint. It is also used as a base layer for gold and silver.
Brushed Nickel : Despite being corrosion-resistant, brushed nickel tarnishes quickly It may get a milky white tarn as it ages and is exposed to the elements. It is used to make kitchen faucets, bathroom fittings, cabinet hardware,lighting fixtures etc.
Power plants and waste incinerators emit nickel into the natural environment. All nickel compounds will, to some degree, adsorb to sediment or soil particles and, therefore, remain sedentary once they are discharged into the environment. However, nickel is bound to get more permeable in the acidic ground and frequently rinse out into the groundwater.




 0086-813-8127573
0086-813-8127573