Number 16917 - Facts about the integer - 16917
By signing up you agree to receive emails from DEWALT® with news, special offers, promotions and other information. You can unsubscribe at any time. See our Privacy Policy or Contact Us by filling out this form, emailing us at support.dewalt@sbdinc.com or sending mail to 701 E. Joppa Road, Towson, Maryland. 21286 for more information.
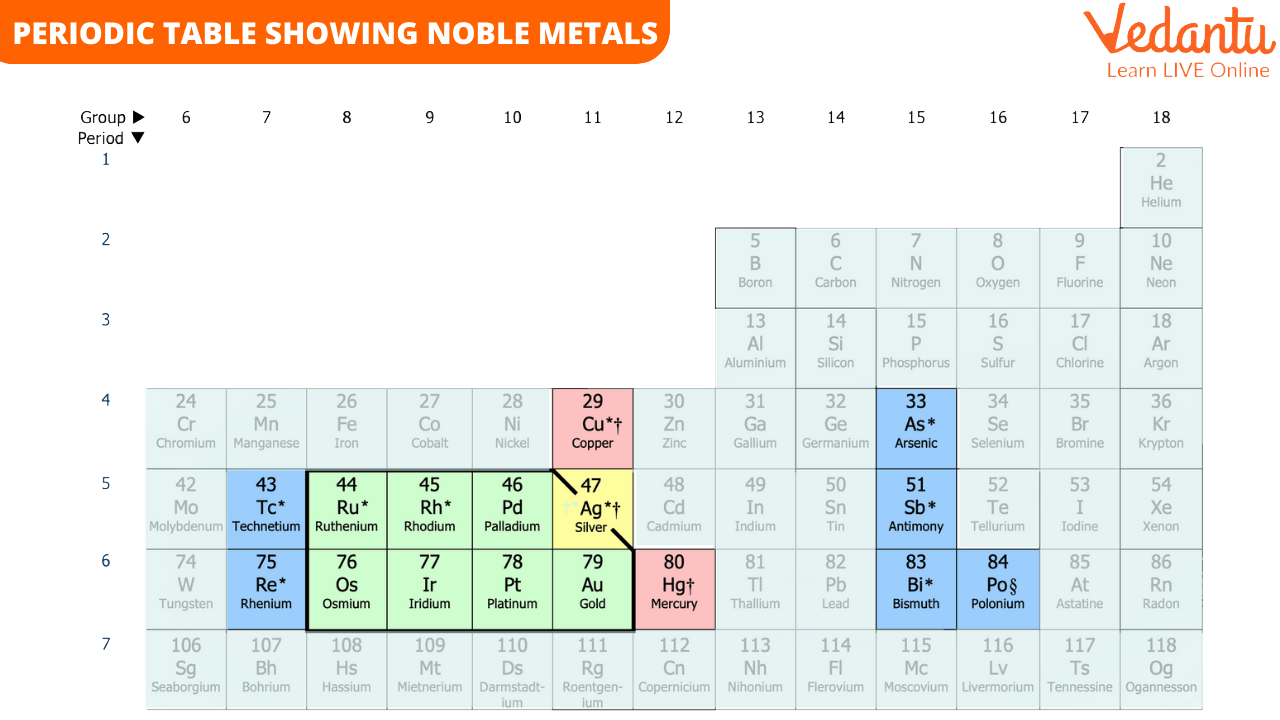
The quality of these metals makes them perfect for various applications. You can see their positions in the periodic table given below. The place of an element in a periodic table also determines the chemical properties of the elements.
Another noble metal used in medicine is rhenium. Rhenium is the last naturally occurring element that has been discovered. It is used to treat liver cancer. Apart from that, it is mixed with nickel so that it can be used in jet engines.
Silver is a malleable metal, but unlike gold, it tarnishes too fast. It is also less expensive than gold. It is one of the noble metals used in medicine in one of its forms; silver nitrate is used as eyedrops for newborn babies to prevent any infections. It's also used for silver plating.
Isn’t it fascinating that such noble metals play an essential role in many processes and are present somewhere in some part of our daily lives? So today, you have learnt everything about noble metals, their types, and their uses. You have learnt that noble metals are the elements that show properties like noble gases, which are resistant to reacting easily. Examples of noble gases are gold, silver, platinum, etc. Noble metals are used in a variety of industries like medicine, dentistry, surgical implants, electroplating, etc.
Noble metals do not react with any “non-noble” elements. They also resist attacks from oxygen or heat, which means their chemical composition doesn’t change. Examples of noble metals are silver, platinum, gold, rhodium, palladium, iridium, osmium, and ruthenium. In simple words, these metals do not rust.
Whenever a non-noble element is burnt in fire or exposed to heat, a certain chemical reaction occurs, and chemicals called oxides are formed. These elements react quickly when exposed to heat and are called “non-noble”. Examples of non-noble elements are calcium, sodium, and magnesium.
By signing up you agree to receive emails from DEWALT® with news, special offers, promotions and other information. You can unsubscribe at any time. See our Privacy Policy or Contact Us by filling out this form, emailing us at support.dewalt@sbdinc.com or sending mail to 6275 Millcreek Drive, Mississauga, ON L5N 7K6, for more information.
Oxidation is the process by which an element reacts with oxygen. Since oxygen is present in the air, metals, when exposed to oxygen or air, form rust or tarnish like iron. Therefore, to protect a metal from oxidation, it is painted with a coating to prevent rust. However, metals like gold and silver do not need much paint as they react less with the oxygen in the air.
The following are trademarks for one or more DEWALT power tools, accessories, anchors and concrete adhesives: The yellow and black color scheme; the “D”-shaped air intake grill; the array of pyramids on the handgrip; the kit box configuration; and the array of lozenge-shaped humps on the surface of the tool.
There are many elements in the last column of the periodic table. These elements placed in the last column are called noble gases due to their inertness or resistance to react with any non-noble element. Due to this property, the metals that show an inert nature or resistance to reaction with other elements are called noble metals. Let's learn more about noble metals and their uses in our lives.
Gold is not only resistant to heat and oxidation from the air but is also malleable, which means that it can be beaten to make thin sheets. It is also ductile, which means it can be stretched to make thin wires. This is why gold is used in microelectronics, where it is used to make leads and wires. Gold is also anti-bacterial; therefore, it is one of the noble metals used in dentistry. However, the price of gold is too high. Commercial uses of this metal are too few, and it's mostly stored as wealth in the form of jewellery or coins.
You must understand that all noble metals are not expensive. Expensive noble metals examples include gold, platinum, and silver. Since you have already learnt that noble metals do not react quickly with other chemicals or elements and do not tarnish or rust, apart from this, the looks and shine of gold, platinum, and silver are excellent and eye-catching. These are the two reasons why noble metals like silver, gold, and platinum are used to make ornaments.
The platinum family includes elements like ruthenium, osmium, rhodium, palladium, etc. These elements are found in Group VIII of the periodic table. These elements come under the "platinum family," as they behave similarly to platinum. Palladium is used in places like electronics and fuel cells. Ruthenium acts as a catalyst for many chemical reactions. Osmium is the highest known element and can be found in surgical implants and the tip of a fountain pen.
Due to the shine and colour of platinum, it is mostly used for making jewellery. Since the metal is durable and resistant to chemical reactions, it is used to make many types of laboratory equipment. It also acts as a catalyst that speeds up a chemical reaction but does not take part in it. Therefore, it is used in the industrial manufacturing of various chemicals like sulfuric acid. Although it is very costly and rare, it is used in places like missiles, jet engines, thermocouple wires, etc. You may be shocked to know that tiny amounts of platinum are present in objects like cigarette lighters, spark plugs, etc.





 0086-813-8127573
0086-813-8127573