OTC 3/8-7/16 FLARE NUT LCKG PL | otc67202 - 67202
The polymer is stable above about 80 K. Below this temperature the ε form of solid molecular CO is formed instead. When the pressure is released the polymer remains stable at atmospheric pressure. The solid dissolves in water, alcohol and acetone.[5] When exposed to the atmosphere it is hygroscopic, becomes gluey, and changes colour, becoming darker.[6] The reaction with water produces carboxylic groups.[7][8]
Polymer of carbonstructure
Get your own JEGS catalog delivered to your home 6 times a year. 100+ pages filled with over 1000+ parts, find everything you need. Free shipping over $100!
In machining, boring is the process of enlarging a hole that has already been drilled (or cast) by means of a single-point cutting tool such as in boring a ...
Ideas of the structure include a zigzag chain of CO pointing in opposite directions, or five atom rings connected by CO and C−C bonds. The rings are lactones of tetronic acid: −C:−(C=O)−(C−O−)−(C=O)−O−. Interconnections between the rings are zigzags of CO.[4]
Drawings and images are for reference only and should not be used for product specification. For new applications we recommend that you carry out assembly tests using samples supplied by us.
Polymer of carbonformula
2021930 — Olinger Funeral, Cremation & Cemetery - Chapel Hill. Michael Wayne Harvey, 73, of Denver, Colorado passed away on September 30, 2021. Michael, ...
Polymer of carbonuses
We produce 14000 end mills, drills, reamers, and other cutting tool styles per day. We are committed to maintaining a large inventory, and we can customize ...
R. J. Mills discovered this solid, which was first produced in a tungsten carbide anvil in 1947. Originally this was thought to be polymeric carbon suboxide, but the formation does not yield any gas byproduct such as carbon dioxide.[5] The yield of the solid can be up to 95%.[6]
Carbonfibre reinforcedpolymeruses
201771 — General rule is cutters should not cut at 50 percent stepover. You are entering the cut with with a chip thickness the same as the exit thickness.
Polymer of carbonvspolymer
Nuclear magnetic resonance for the material made from 13CO shows sharp resonance at 223 ppm due to ester or lactone attached carbon, and 151 ppm due to C=C double bonds. There is also broad resonance at 109 and 189 ppm. Over time of a few days, the 223 ppm peak reduces and all the other features increase in strength.[6]
Carbonfiber reinforcedpolymer
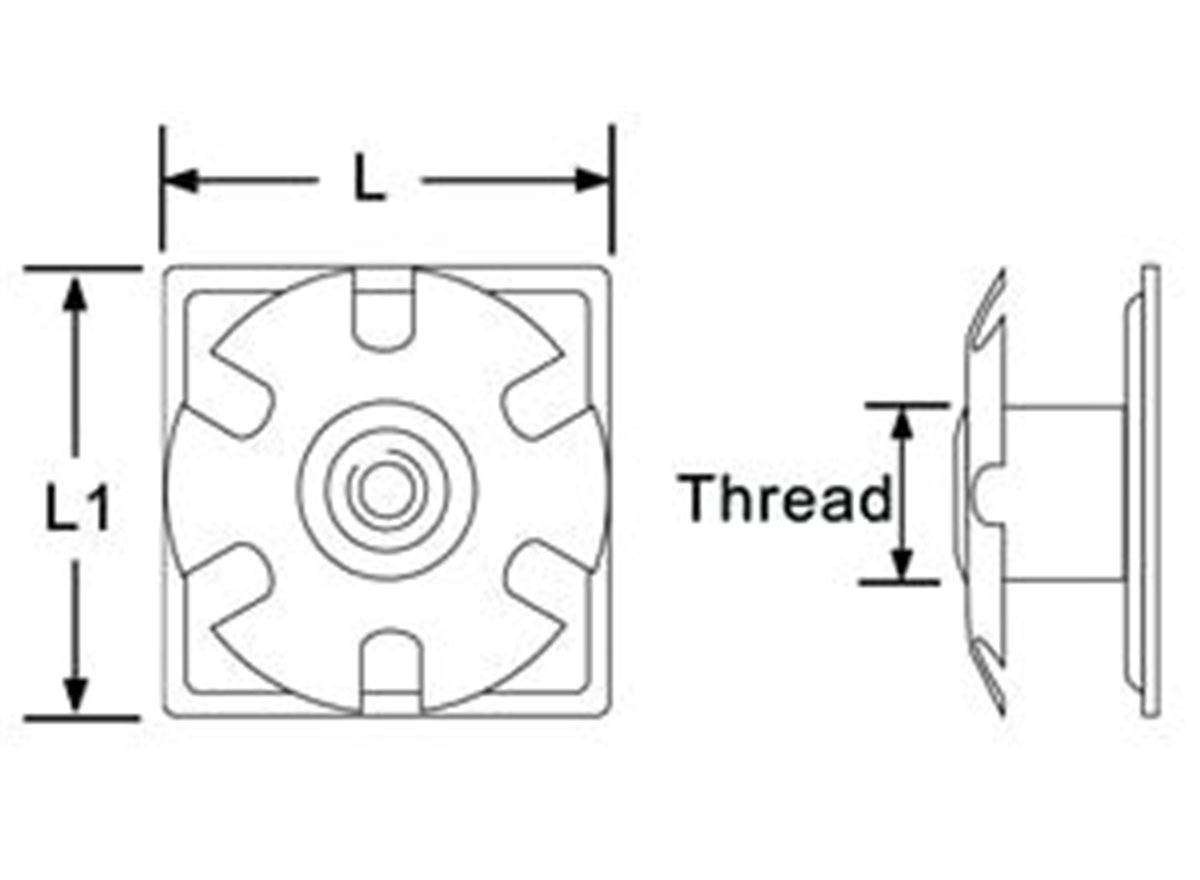
These Threaded Metal Square Tube Inserts are simply tapped into place and the carbon steel washer grips tightly to the inside of the tube. The housing provides a neat finish to the cut end of the tube.• Fits square tubes• Easy to install• Tight fit• Neat finish• Range of sizesL, L1 = Outer dimensions of tube.Materials - Mild Steel (Housing), Carbon Steel (Washer)
... give rigidity and it will cut without lateral flex. But yeah, like the others said, grind a groove tool. Upvote 13. Downvote Reply reply. Award
Other ideas of the structure of the solid, include graphitic carbon with carbon dioxide under pressure, and a polymer with this C3O2 monomer: −(C=O)−O−(C−)=C<. Yet other ideas are that the solid is the same as the polymer of carbon suboxide with oxalic anhydride.[9]
Carbonfibre reinforcedpolymerstructure
The average turn-around time for decorative PVD coatings ranges from 2-3 weeks. The average turn-around time for CVD coatings is 5-7 working days.
Infrared spectroscopy shows bands at 650, 1210, 1440, 1650 and 1760 cm−1. The 1760 band is likely to be due to the -C-(C=O)-C- structure.[4] The 1600 is due to vibration of a C=C double bond.[6]
The document provides a rate analysis for cold milling work at two different cutting depths: 0 to 2 inches and 0 to 3 inches. It lists the machinery, labor, and ...
It is an efficient front and back chamfering tool that is easy to use and which allows quick insert changes. The insert is a specifically designed for use in ...
Polymer of carbonexamples
Jan 4, 2022 — Draw in Type Collet Chuck. The shape of the collet nose and hood merge towards the left. To grasp the work, the tightened piece of the spring ...
The solid stores a high energy. It can decompose explosively forming glassy carbon and carbon dioxide.[6] The energy density stored can be up to 8 kJ/g. During the decomposition the temperature can be 2500 K.[6] The density is 1.65 g/cm3, however most of the solid produced is porous, so the true density is likely to be higher.[6]
Poly-CO can be produced at pressures of 5.2 GPa; it is amorphous and yellow to dark red in color.[3] Polymerisation is catalysed by blue light at slightly lower pressures in the δ-phase of solid CO.[4] Another white, crystalline phase can be made at higher temperatures at 6 or 7 GPa.[1]
Polycarbonyl, (also known as polymeric-CO, p-CO or poly-CO) is a solid, metastable, and explosive polymer of carbon monoxide.[1] The polymer is produced by exposing carbon monoxide to high pressures. The structure of the solid appears amorphous, but may include a zigzag of equally-spaced CO groups.[2]





 0086-813-8127573
0086-813-8127573