Tap & Die Sets - speed taps canada
Hardenability of steelpdf
B. M. Larsen and T. E. Brower, “Critical Studies of a Modified Ledebur Method for Determination of Oxygen in Steel,” Transactions, American Institute of Mining and Metallurgical Engineers, 1932, Vol. 100, Iron and Steel Division, p. 196–227.

TUNGALOY CORPORATION 11-1 Yoshima-Kogyodanchi, Iwaki, Fukushima, 970-1144 Japan Phone: +81-246-36-8501 Fax: +81-246-36-8542 CONTACT FORM >
Hardenability of steelchart
G. V. Luerssen, “Some Notes on the Behavior of Carbon Tool Steel on Quenching,”Transactions, American Society for Steel Treating, Feb. 1930, Vol. XVII, p. 161–192.
Hardenabilityvs hardness
Henry Marion Howe Memorial Lecture, 1932, Edgar C. Bain, “On the Rates of Reactions in Solid Steel,” Transactions, American Institute of Mining and Metallurgical Engineers, 1932, Vol. 100, Iron and Steel Division, p. 13–46.
Bain, E.C. Factors affecting the inherent hardenability of steel. J. Heat Treating 1, 57–100 (1979). https://doi.org/10.1007/BF02833240
S. Epstein and H. S. Rawdon, “Progress in Study of Normal and Abnormal Steel,”Transactions, American Society for Steel Treating, Sept. 1927, Vol. XII, p. 337–375.

Hardenability of steelcalculator
This maximum rate of austenite transformation to products other than martensite is in turn largely determined by the condition of the austenite at the moment of quenching with respect to two factors:
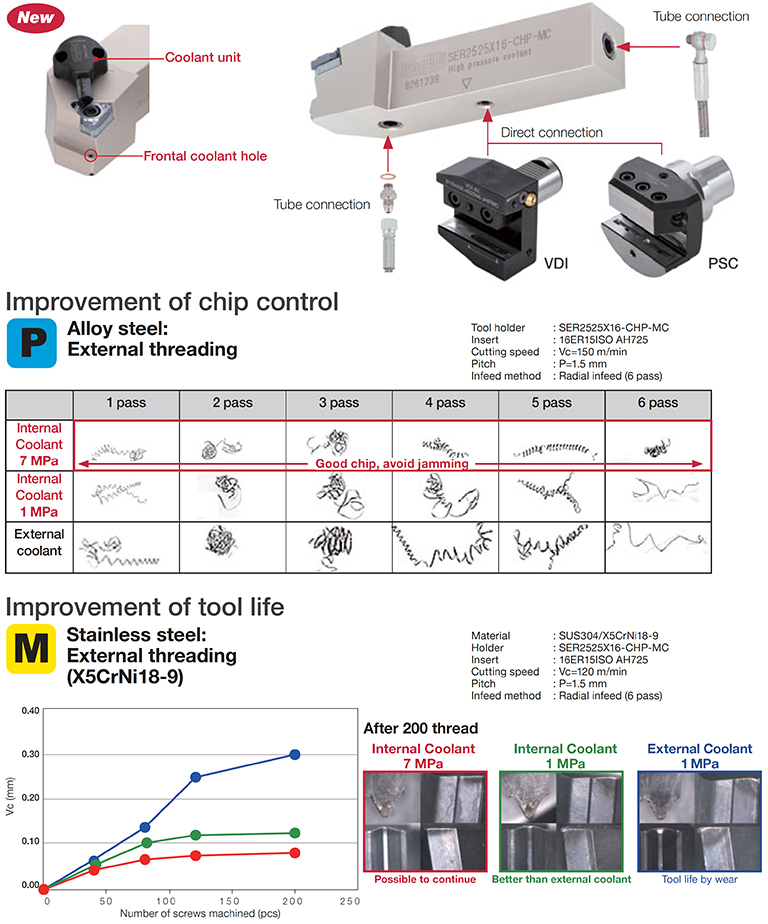
• Optimized two streams of high-pressure coolant jets to maximize productivity. • Applicable direct connection with tube free.
Hardenability of steeldepends on
F. F. Lucas, Henry Marion Howe Memorial Lecture, 1931, “On the Art of Metallography,” Transactions, American Institute of Mining and Metallurgical Engineers, 1931, Iron and Steel Division, p. 11–44.
its effective grain size. The finer the grain, the more rapid is the transformation to fine pearlite, and correspondingly the lower is the hardenability. Effective grain size seems to be the most potent single factor influencing hardenability; it in turn is probably controlled largely, for any specified temperature, by the obstruction to grain growth offered by large numbers of very finely dispersed particles, of exceedingly small aggregate mass, comprised presumably of stable oxides such as alumina, vanadia, and probably silica or silicates.
its composition with respect to dissolved elements, most of which retard, though some may hasten, its transformation to pearlite. Manganese, chromium, nickel, silicon, and aluminum definitely retard the reaction and so contribute directly to deep hardening about in the order named; on the other hand, tungsten, cobalt, molybdenum, vanadium, and possibly oxygen appear to induce shallow hardening, though most probably indirectly by restricting grain growth.
The hardenability of a steel depends upon the actual rate at which its austenite transforms to fine pearlite at the particular temperature at which this reaction sets in most promptly. The precise temperature at which this transformation rate is greatest depends upon the composition of the steel; in any case, it is this maximum rate at temperatures near 950 degrees Fahr. (500 degrees Cent.), which determines the critical quenching speed which must be exceeded if the steel is to be hardened.
H. W. McQuaid and E. W. Ehn, “Effect of Quality of Steel on Case-carburizing Results,”Transactions, American Institute of Mining and Metallurgical Enginers, 1922, Vol. 67, p. 341–91.




 0086-813-8127573
0086-813-8127573